A Randomized, placebo-controlled, crossover pilot trial of the oral selective NR2B antagonist MK-0657 in patients with treatment-resistant major depressive disorder - PubMed (original) (raw)
Randomized Controlled Trial
A Randomized, placebo-controlled, crossover pilot trial of the oral selective NR2B antagonist MK-0657 in patients with treatment-resistant major depressive disorder
Lobna Ibrahim et al. J Clin Psychopharmacol. 2012 Aug.
Abstract
Converging lines of evidence suggest that the glutamatergic system may play an increasingly important role in the development of novel therapeutics for major depressive disorder (MDD), particularly agents associated with rapid antidepressant effects. Diverse glutamatergic modulators targeting N-methyl-D-aspartate receptors have shown efficacy in MDD, but their associated psychotomimetic effects presently preclude their use in larger samples. This small, randomized, double-blind, placebo-controlled, crossover pilot study evaluated the potential antidepressant efficacy and tolerability of an oral formulation of the selective N-methyl-D-aspartate NR2B antagonist MK-0657 in patients with treatment-resistant MDD (TRD). The TRD subjects underwent a 1-week drug-free period and were subsequently randomized to receive either MK-0657 monotherapy (4-8 mg/d) or placebo for 12 days. Because of recruitment challenges and the discontinuation of the compound's development by the manufacturer, only 5 of the planned 21 patients completed both periods of the crossover administration of MK-0657 and placebo. Significant antidepressant effects were observed as early as day 5 in patients receiving MK-0657 compared with those receiving placebo, as assessed by the Hamilton Depression Rating Scale and Beck Depression Inventory; however, no improvement was noted when symptoms were assessed with the Montgomery-Asberg Depression Rating Scale, the primary efficacy measure. No serious or dissociative adverse effects were observed in patients receiving this oral formulation of MK-0657. Despite the small sample size, this pilot study suggests that an oral formulation of the NR2B antagonist MK-0657 may have antidepressant properties in TRD patients. Further studies with larger sample sizes are necessary to confirm these preliminary findings.
Trial registration: ClinicalTrials.gov NCT00472576.
Figures
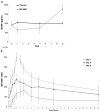
FIGURE 1
Changes in plasma BDNF (A) and MK-0657 levels (B) during 9 days of treatment with MK-0657. The BDNF levels were evaluated at baseline and on days 1, 5, and 9 of treatment in the active drug compared with placebo. Blood samples for MK-0657 pharmacokinetics were collected predose and at 0.5, 1, 1.5, 2, 3, 4, 6, 8, 12, 18, and 24 hours after dose on days 1, 5, 9. *P < 0.05.
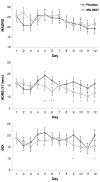
FIGURE 2
Change in the 17-item Hamilton Depression Rating Scale (HAM-D) scores, Beck Depression Inventory (BDI) scores, and Montgomery-Asberg Depression Rating Scale (MADRS) scores in drug-free TRD subjects followed receiving 12 days of MK-0657 compared with placebo. *P < 0.05.
Similar articles
- An innovative design to establish proof of concept of the antidepressant effects of the NR2B subunit selective N-methyl-D-aspartate antagonist, CP-101,606, in patients with treatment-refractory major depressive disorder.
Preskorn SH, Baker B, Kolluri S, Menniti FS, Krams M, Landen JW. Preskorn SH, et al. J Clin Psychopharmacol. 2008 Dec;28(6):631-7. doi: 10.1097/JCP.0b013e31818a6cea. J Clin Psychopharmacol. 2008. PMID: 19011431 Clinical Trial. - A Randomized Trial of the N-Methyl-d-Aspartate Receptor Glycine Site Antagonist Prodrug 4-Chlorokynurenine in Treatment-Resistant Depression.
Park LT, Kadriu B, Gould TD, Zanos P, Greenstein D, Evans JW, Yuan P, Farmer CA, Oppenheimer M, George JM, Adeojo LW, Snodgrass HR, Smith MA, Henter ID, Machado-Vieira R, Mannes AJ, Zarate CA. Park LT, et al. Int J Neuropsychopharmacol. 2020 Jul 29;23(7):417-425. doi: 10.1093/ijnp/pyaa025. Int J Neuropsychopharmacol. 2020. PMID: 32236521 Free PMC article. Clinical Trial. - A randomized trial of a low-trapping nonselective N-methyl-D-aspartate channel blocker in major depression.
Zarate CA Jr, Mathews D, Ibrahim L, Chaves JF, Marquardt C, Ukoh I, Jolkovsky L, Brutsche NE, Smith MA, Luckenbaugh DA. Zarate CA Jr, et al. Biol Psychiatry. 2013 Aug 15;74(4):257-64. doi: 10.1016/j.biopsych.2012.10.019. Epub 2012 Dec 1. Biol Psychiatry. 2013. PMID: 23206319 Free PMC article. Clinical Trial. - A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression.
Zarate CA Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, Charney DS, Manji HK. Zarate CA Jr, et al. Arch Gen Psychiatry. 2006 Aug;63(8):856-64. doi: 10.1001/archpsyc.63.8.856. Arch Gen Psychiatry. 2006. PMID: 16894061 Clinical Trial. - Intravenous ketamine for treatment-resistant major depressive disorder.
Covvey JR, Crawford AN, Lowe DK. Covvey JR, et al. Ann Pharmacother. 2012 Jan;46(1):117-23. doi: 10.1345/aph.1Q371. Epub 2011 Dec 20. Ann Pharmacother. 2012. PMID: 22190250 Review.
Cited by
- Mood Therapeutics: Novel Pharmacological Approaches for Treating Depression.
Henter ID, de Sousa RT, Gold PW, Brunoni AR, Zarate CA Jr, Machado-Vieira R. Henter ID, et al. Expert Rev Clin Pharmacol. 2017 Feb;10(2):153-166. doi: 10.1080/17512433.2017.1253472. Epub 2017 Jan 16. Expert Rev Clin Pharmacol. 2017. PMID: 27781556 Free PMC article. Review. - Alternatives to ketamine in depression: state-of-the-art and future perspectives.
Jelen LA, King S, Stone JM. Jelen LA, et al. Ther Adv Psychopharmacol. 2018 Mar;8(3):95-98. doi: 10.1177/2045125317749456. Epub 2017 Dec 18. Ther Adv Psychopharmacol. 2018. PMID: 29492257 Free PMC article. No abstract available. - Extrasynaptic CaMKIIα is involved in the antidepressant effects of ketamine by downregulating GluN2B receptors in an LPS-induced depression model.
Tang XH, Zhang GF, Xu N, Duan GF, Jia M, Liu R, Zhou ZQ, Yang JJ. Tang XH, et al. J Neuroinflammation. 2020 Jun 10;17(1):181. doi: 10.1186/s12974-020-01843-z. J Neuroinflammation. 2020. PMID: 32522211 Free PMC article. - Rodent models of treatment-resistant depression.
Caldarone BJ, Zachariou V, King SL. Caldarone BJ, et al. Eur J Pharmacol. 2015 Apr 15;753:51-65. doi: 10.1016/j.ejphar.2014.10.063. Epub 2014 Nov 21. Eur J Pharmacol. 2015. PMID: 25460020 Free PMC article. Review.
References
- Trivedi MH, Rush AJ, Wisniewski SR, et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry. 2006;163(1):28–40. - PubMed
- Jick H, Kaye JA, Jick SS. Antidepressants and the risk of suicidal behaviors. JAMA. 2004;292(3):338–343. - PubMed
- Berman RM, Cappiello A, Anand A, et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47(4):351–354. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical