New insights into nucleosome and chromatin structure: an ordered state or a disordered affair? - PubMed (original) (raw)
Review
New insights into nucleosome and chromatin structure: an ordered state or a disordered affair?
Karolin Luger et al. Nat Rev Mol Cell Biol. 2012.
Abstract
The compaction of genomic DNA into chromatin has profound implications for the regulation of key processes such as transcription, replication and DNA repair. Nucleosomes, the repeating building blocks of chromatin, vary in the composition of their histone protein components. This is the result of the incorporation of variant histones and post-translational modifications of histone amino acid side chains. The resulting changes in nucleosome structure, stability and dynamics affect the compaction of nucleosomal arrays into higher-order structures. It is becoming clear that chromatin structures are not nearly as uniform and regular as previously assumed. This implies that chromatin structure must also be viewed in the context of specific biological functions.
Conflict of interest statement
Competing interests statement
The authors declare no competing financial interests.
Figures
Figure 1. Primary, secondary and tertiary structure of chromatin
The primary structure is shown as nucleosomal arrays consisting of nucleosomes with canonical histones (shown in light blue and yellow) and combinations of different histone variants (shown in green, purple and light blue). Nucleosomes with canonical or histone variants may vary in the degree of post-translational modifications (PTMs; such as acetylation, methylation, phosphorylation, ubiquitylation and sumoylation), generating the possibility for nucleosomes with a large number of different ‘colours’. Histone variants and PTMs may affect nucleosome structure and dynamics. The spacing between nucleosomes may vary on the basis of the underlying sequence, action of chromatin-remodelling enzymes and DNA binding by other factors (for example, transcription activators). Short-range nucleosome–nucleosome interactions result in folded chromatin fibres (secondary chromatin structure, lower left panel). Fibre–fibre interactions, which are defined by long-range interactions between individual nucleosomes, are also affected by the primary structure of chromatin fibres, including PTMs, histone variants and spacing of nucleosomes. Secondary and tertiary structures are stabilized by architectural proteins, such as linker histone H1, methyl-CpG-binding protein 2 (MeCP2), heterochromatin protein 1 (HP1), high mobility group (HMG) proteins, poly(ADP-ribose) polymerase 1 (PARP1), myeloid and erythroid nuclear termination stage-specific protein (MENT), Polycomb group proteins and many others. Transitions between the different structural states are indicated by double arrows; these may be regulated by changes in patterns of PTMs, binding or displacement of architectural proteins, exchange of histone variants and chromatin-remodelling factors.
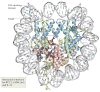
Figure 2. Nucleosome structure and the acidic patch: a common interaction interface for many nucleosome-interacting proteins
The structure of the nucleosome (Protein Data Bank code 1AOI) is viewed down the superhelical axis of the DNA. Histones H3, H4, H2A and H2B are shown in light blue, green, yellow and red, respectively. The figure indicates the amino-terminal α-helix of H3 (H3αN), which organizes the penultimate 10 bp of the DNA, and the carboxy-terminal end of the H2A docking domain. Acidic residues on H2A and H2B (the ‘acidic patch’) that are involved in the interaction with the H4 tail and with nucleosome-interacting proteins (such as the latency-associated nuclear antigen (LANA) peptide, interleukin-33 (IL-33), regulator of chromosome condensation 1 (RCC1), silent information regulator 3 (Sir3) and high mobility group nucleosome-binding domain-containing protein 2 (HMGN2)) are indicated in bright red; additional residues that are implicated in the interaction interfaces with the proteins listed above are shown in dark blue. Note that the number of total histone residues implicated in all of these protein–protein interfaces is relatively small, and that all cluster in a contained region on the surface of the histone octamer. In the absence of these factors, the interaction of the H4 tail from a neighbouring particle with the acidic patch mediates nucleosome–nucleosome interactions, thereby promoting chromatin folding. By using similar interactions with acidic patch on the nucleosome, the proteins listed above may compete with the H4 tail and modulate chromatin structure.
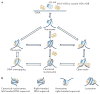
Figure 3. The many structural states of the nucleosome
a | Structural states of the nucleosome that are likely to be interchangeable. These include the tetrasome, which is formed by the wrapping of ~80 bp DNA around an (H3–H4)2 tetramer. Hexasomes (nucleosomes lacking one H2A–H2B heterodimer) are intermediate states during nucleosome assembly or disassembly, as well as during transcription of nucleosome templates by RNA polymerase II. Both hexasomes and fully formed nucleosomes may undergo spontaneous structural transitions that are characterized either by the transient release of the DNA ends (DNA breathing) or by a transient opening of the interface between histone subcomplexes (open state). Some of the specific states may be favoured by DNA sequence, histone variant incorporation or post-translational modifications (PTMs). Histone variants are likely to be incorporated by similar pathways. b | Nucleosomes may also exist in alternative states that vary in the direction of the handedness of the DNA superhelix (left-handed versus right-handed) or in the stoichiometry (hemisome) and structural states (lexosome) of histones. These alternative structures, if they do indeed exist in vivo, would affect DNA accessibility and the interaction of nuclear factors with chromatin.
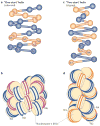
Figure 4. Two models for chromatin secondary structure
The solenoid model is characterized by interactions between consecutive nucleosomes (n, n + 1; a,b), whereas the zigzag model implies interactions between alternate nucleosomes (n, n + 2; c,d). The alternative nucleosomes are numbered from N1 to N8. In the solenoid model proposed by Rhodes and colleagues, the 30 nm chromatin fibre is an interdigitated one-start helix in which a nucleosome in the fibre interacts with its fifth and sixth neighbour nucleosomes. Alternative helical gyres are coloured blue and magenta (b). In the zigzag model, the chromatin fibre is a two-start helix in which nucleosomes are arranged in a zigzag manner such that a nucleosome in the fibre binds to the second neighbour nucleosome. Alternative nucleosome pairs are coloured blue and orange (d). The two models also differ in the trajectory and degree of bending of the DNA that connects two nucleosomes (linker DNA). Figure is reproduced, with permission, from REF. © (2011) Elsevier.
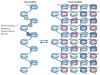
Figure 5. Nucleosome–nucleosome interactions mediated by histone tails and the nucleosomal surface
a | Nucleosome–nucleosome interactions in the crystal structures of unconnected 147 bp nucleosomes (Protein Data Bank (PDB) code 1AOI).b | Nucleosome– nucleosome interactions, as seen in the low-resolution tetranucleosome structure of four connected nucleosomes (PDB code 1ZBB; only two nucleosomes are shown). The resolution of this structure does not reveal the location of the H4 tail. A cartoon at the bottom of each panel shows the relative arrangement of the two nucleosomes with respect to each other. Residues constituting the acidic patch are shown in red, and the H4 tail (in a) is shown in green; it is not resolved in the structures shown in b.
Figure 6. A model for chromatin tertiary structure by interdigitation of nucleosomal arrays
The dynamic motion of arrays, possibly aided by nucleosome remodelling factors and histone-modifying enzymes, enables partial decompaction by the ‘unpeeling’ of an unfolded array (light blue) from the large-scale interdigitated tertiary chromatin fibre. As a result, this ‘string of pearls’ (shown on the left) slides out of the compact array and becomes more accessible as it is removed from the interdigitated stacks (shown on the right). This unpeeling process from the surface of the fibre facilitates transcription factor access. The fractal nature of the interdigitated chromatin fibre enables access to even DNA that is buried deep within the fibre.
Similar articles
- HMGN1 and 2 remodel core and linker histone tail domains within chromatin.
Murphy KJ, Cutter AR, Fang H, Postnikov YV, Bustin M, Hayes JJ. Murphy KJ, et al. Nucleic Acids Res. 2017 Sep 29;45(17):9917-9930. doi: 10.1093/nar/gkx579. Nucleic Acids Res. 2017. PMID: 28973435 Free PMC article. - Structures of chromatin modulators in complex with nucleosome.
Min J, Liu K. Min J, et al. Curr Opin Chem Biol. 2021 Aug;63:105-114. doi: 10.1016/j.cbpa.2021.02.018. Epub 2021 Apr 3. Curr Opin Chem Biol. 2021. PMID: 33823458 Review. - Structural dynamics of nucleosome core particle: comparison with nucleosomes containing histone variants.
Ramaswamy A, Bahar I, Ioshikhes I. Ramaswamy A, et al. Proteins. 2005 Feb 15;58(3):683-96. doi: 10.1002/prot.20357. Proteins. 2005. PMID: 15624215 - Global dynamics of newly constructed oligonucleosomes of conventional and variant H2A.Z histone.
Ramaswamy A, Ioshikhes I. Ramaswamy A, et al. BMC Struct Biol. 2007 Nov 8;7:76. doi: 10.1186/1472-6807-7-76. BMC Struct Biol. 2007. PMID: 17996059 Free PMC article. - Post-translational modifications and chromatin dynamics.
Tolsma TO, Hansen JC. Tolsma TO, et al. Essays Biochem. 2019 Apr 23;63(1):89-96. doi: 10.1042/EBC20180067. Print 2019 Apr 23. Essays Biochem. 2019. PMID: 31015385 Review.
Cited by
- Testis-specific H2B.W1 disrupts nucleosome integrity by reducing DNA-histone interactions.
Ding D, Pang MYH, Deng M, Nguyen TT, Liu Y, Sun X, Xu Z, Zhang Y, Zhai Y, Yan Y, Ishibashi T. Ding D, et al. Nucleic Acids Res. 2024 Oct 28;52(19):11612-11625. doi: 10.1093/nar/gkae825. Nucleic Acids Res. 2024. PMID: 39329259 Free PMC article. - Heterochromatin protein 1 alpha (HP1α) undergoes a monomer to dimer transition that opens and compacts live cell genome architecture.
Lou J, Deng Q, Zhang X, Bell CC, Das AB, Bediaga NG, Zlatic CO, Johanson TM, Allan RS, Griffin MDW, Paradkar P, Harvey KF, Dawson MA, Hinde E. Lou J, et al. Nucleic Acids Res. 2024 Oct 14;52(18):10918-10933. doi: 10.1093/nar/gkae720. Nucleic Acids Res. 2024. PMID: 39193905 Free PMC article. - Ubiquitinated histone H2B as gatekeeper of the nucleosome acidic patch.
Hicks CW, Rahman S, Gloor SL, Fields JK, Husby NL, Vaidya A, Maier KE, Morgan M, Keogh MC, Wolberger C. Hicks CW, et al. Nucleic Acids Res. 2024 Sep 9;52(16):9978-9995. doi: 10.1093/nar/gkae698. Nucleic Acids Res. 2024. PMID: 39149911 Free PMC article. - Multifunctional histone variants in genome function.
Wong LH, Tremethick DJ. Wong LH, et al. Nat Rev Genet. 2024 Aug 13. doi: 10.1038/s41576-024-00759-1. Online ahead of print. Nat Rev Genet. 2024. PMID: 39138293 Review. - Determining mesoscale chromatin structure parameters from spatially correlated cleavage data using a coarse-grained oligonucleosome model.
Clerkin AB, Pagane N, West DW, Spakowitz AJ, Risca VI. Clerkin AB, et al. bioRxiv [Preprint]. 2024 Jul 29:2024.07.28.605011. doi: 10.1101/2024.07.28.605011. bioRxiv. 2024. PMID: 39131347 Free PMC article. Preprint.
References
- Luger K, Mader AW, Richmond RK, Sargent DF, Richmond TJ. Crystal structure of the nucleosome core particle at 2.8 A resolution. Nature. 1997;389:251–260. This is the first structure of the nucleosome that detailed the interactions between DNA and histones as well as among histone proteins at nearly atomic resolution. - PubMed
- Tremethick DJ. Higher-order structures of chromatin: the elusive 30 nm fiber. Cell. 2007;128:651–654. - PubMed
- Woodcock CL, Dimitrov S. Higher-order structure of chromatin and chromosomes. Curr Opin Genet Dev. 2001;11:130–135. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources