Stonin 2 is a major adaptor protein for clathrin-mediated synaptic vesicle retrieval - PubMed (original) (raw)
Stonin 2 is a major adaptor protein for clathrin-mediated synaptic vesicle retrieval
Anna K Willox et al. Curr Biol. 2012.
Abstract
At small synapses in the brain, clathrin-mediated endocytosis (CME) is the dominant mode of synaptic vesicle retrieval following weak stimulation [1-4]. Clathrin cannot bind to membranes or cargo directly and instead uses adaptor proteins to do so [5]. Although the involvement of clathrin and dynamin in synaptic vesicle retrieval is clear, it is unknown which adaptor proteins are used to sort the essential components into the vesicle [1, 4, 6]. In nonneuronal cells, CME of the majority of transmembrane receptors is either directly or indirectly via the heterotetrameric AP-2 complex [5]. In neurons, RNAi of the μ2 subunit of AP-2 resulted in only minor inhibition of synaptic vesicle retrieval [7, 8], a result echoed in C. elegans [9]. These results suggest that alternative adaptors may be employed for vesicle retrieval. Here, we tested which adaptors are required for vesicle retrieval at hippocampal synapses using a targeted RNAi screen coupled with optical measurements. Stonin 2 emerged as a major adaptor, whereas AP-2 played only a minor role in endocytosis at the synapse. Moreover, using chemically induced rerouting of stonin 2 to mitochondria it was possible to switch endocytically competent synapses to an impaired state on a timescale of minutes.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures
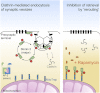
Graphical abstract
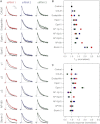
Figure 1
Targeted RNAi Screen to Identify Adaptor Proteins for Clathrin-Mediated Synaptic Vesicle Endocytosis (A) Average fluorescence traces of synapses expressing synaptophysin-pHluorin (sypHy). Three siRNAs per target are shown compared to control GL2 siRNA (black). In all figures (unless indicated otherwise) responses to stimulation with 40 APs at 20 Hz are mean ± SEM, normalized to allow direct comparison of fluorescence decay. Overlaid is a fit to a function that describes retrieval (see Supplemental Experimental Procedures). (B) Summary of the relative rates of endocytosis for each condition in the screen. The time taken for fluorescence to reach 1/e of its poststimulus value (T1/e) is shown normalized to control siRNA. (C) Summary of the average exocytic response to 40 APs at 20 Hz. Mean ± SEM responses of sypHy traces under the conditions of the screen are shown normalized to control siRNA. Colors in (B and C) correspond to the siRNAs in (A). Nsynapse = 91–534, Nneuron = 3–10 from 3–6 independent cultures. See also Figure S1.
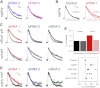
Figure 2
AP-2 Is Not Essential for Synaptic Vesicle Retrieval (A–C) Average fluorescence traces of synapses expressing sypHy. Three siRNAs per target are shown compared to control GL2 siRNA (black). Schematic diagrams (left) show the subunit(s) targeted by RNAi (light gray). (A) Depletion of α, β2, or σ2 subunits. (B) Hemicomplex depletion of α and σ2 or β2 and μ2 subunits. Here, siRNA 1 of α or β2 was cotransfected with one of three siRNAs targeting σ2 or μ2, respectively. (C) Depletion of μ2 subunit of AP-2 for 96 hr. Nsynapse = 107–451, Nneuron = 3–10 from 2–5 independent cultures. (D) Summary of the relative rates of endocytosis for each condition in this figure. T1/e is shown normalized to control siRNA. (E) Representative confocal micrographs showing the uptake of Alexa 488-transferrin (50 μg/ml for 10 min at 37°C) in neurons expressing mCherry that were cotransfected with control or AP-2 (μ2) siRNA. Scale bar represents 20 μm. Bar chart to show the quantification of fluorescent transferrin uptake at neuronal soma for mCherry-expressing neurons cotransfected with control (GL2), clathrin heavy chain, or μ2 siRNA. ∗∗p < 0.01 Kruskal-Wallis test.
Figure 3
Stonin 2 Is a Major Adaptor for Synaptic Vesicle Endocytosis (A) Average fluorescence traces of synapses expressing sypHy. Two further siRNAs designed to target stonin 2 are shown compared to control GL2 siRNA. (B) Rescue of endocytic defects caused by stonin 2-depletion. SypHy responses were measured in control or stonin 2-depleted cultures expressing either mCherry or mCherry-Stonin 2 as indicated. (C) Average fluorescence traces of synapses expressing vGlut1-pHluorin (above) or synaptotagmin 1-pHluorin (below). Three stonin 2 siRNAs are shown compared to control siRNA (black). Nsynapse = 49–338, Nneuron = 3–11 from 2–7 independent cultures. (D) Average fluorescence traces of synapses expressing sypHy. Three stonin 2 siRNAs are shown compared to control siRNA (black). Responses to stimulation with 10 APs at 20 Hz are shown (D only). Nsynapse = 25–48, Nneuron = 2 from one experiment. (E) Summaries of the relative rates of endocytosis for each condition in this figure. T1/e is shown normalized to control GL2 siRNA + mCherry (bar chart) or control GL2 siRNA (dot plot).
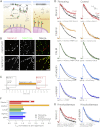
Figure 4
Rapid, Chemically Induced Rerouting of Stonin 2 to Mitochondria (A) Schematic diagram to show the rerouting method [20]. A presynaptic terminal expressing MitoTrap (mito-XFP-FRB) and mCherry-FKBP-stonin 2 is shown. Rapamycin binds the FKBP and FRB domains tightly so that its application causes dimerization of MitoTrap and mCherry-FKBP-stonin 2. (B) Representative confocal images from fixed cultures to show rapamycin-dependent rerouting of mCherry-FKBP-Stonin 2 to mitochondria expressing MitoTrap (mito-YFP-FRB). Scale bar represents 10 μm. (C) Experimental protocol showing the two imaging periods (pre and post), stimulations, and rapamycin application. (D) Effect on sypHy retrieval of rerouting stonin 2, clathrin, CALM, or AP-2. FKBP-tagged contructs (rerouting) are shown to the left and those without FKBP (control) to the right. Average sypHy fluorescence traces of synapses expressing sypHy and MitoTrap (mito-PAGFP-FRB) together with the indicated construct for rerouting. (E) Knocksideways experiments were performed as described for rerouting but with the concomitant depletion of stonin 2 or σ2. In (D) and (E), the first stimulation (40 AP 20 Hz) is shown in black (pre) and the second following rapamycin application (1 μM, 6 min) is shown in a different color (post). Nsynapse = 56–455, Nneuron = 2–9 from 1–7 independent cultures. (F) Summary of the relative rates of endocytosis for each condition in this figure. T1/e is shown normalized to the prerapamycin recovery. See also Figure S2.
Similar articles
- Clathrin/AP-2 mediate synaptic vesicle reformation from endosome-like vacuoles but are not essential for membrane retrieval at central synapses.
Kononenko NL, Puchkov D, Classen GA, Walter AM, Pechstein A, Sawade L, Kaempf N, Trimbuch T, Lorenz D, Rosenmund C, Maritzen T, Haucke V. Kononenko NL, et al. Neuron. 2014 Jun 4;82(5):981-8. doi: 10.1016/j.neuron.2014.05.007. Neuron. 2014. PMID: 24908483 - A distributed set of interactions controls mu2 functionality in the role of AP-2 as a sorting adaptor in synaptic vesicle endocytosis.
Kim SH, Ryan TA. Kim SH, et al. J Biol Chem. 2009 Nov 20;284(47):32803-12. doi: 10.1074/jbc.M109.039149. Epub 2009 Sep 17. J Biol Chem. 2009. PMID: 19762466 Free PMC article. - Stonin 2 is an AP-2-dependent endocytic sorting adaptor for synaptotagmin internalization and recycling.
Diril MK, Wienisch M, Jung N, Klingauf J, Haucke V. Diril MK, et al. Dev Cell. 2006 Feb;10(2):233-44. doi: 10.1016/j.devcel.2005.12.011. Dev Cell. 2006. PMID: 16459302 - Stonins--specialized adaptors for synaptic vesicle recycling and beyond?
Maritzen T, Podufall J, Haucke V. Maritzen T, et al. Traffic. 2010 Jan;11(1):8-15. doi: 10.1111/j.1600-0854.2009.00971.x. Traffic. 2010. PMID: 19732400 Review. - Molecular mechanism and physiological functions of clathrin-mediated endocytosis.
McMahon HT, Boucrot E. McMahon HT, et al. Nat Rev Mol Cell Biol. 2011 Jul 22;12(8):517-33. doi: 10.1038/nrm3151. Nat Rev Mol Cell Biol. 2011. PMID: 21779028 Review.
Cited by
- Sensing Exocytosis and Triggering Endocytosis at Synapses: Synaptic Vesicle Exocytosis-Endocytosis Coupling.
Lou X. Lou X. Front Cell Neurosci. 2018 Mar 14;12:66. doi: 10.3389/fncel.2018.00066. eCollection 2018. Front Cell Neurosci. 2018. PMID: 29593500 Free PMC article. Review. - ENTH and ANTH domain proteins participate in AP2-independent clathrin-mediated endocytosis.
Manna PT, Gadelha C, Puttick AE, Field MC. Manna PT, et al. J Cell Sci. 2015 Jun 1;128(11):2130-42. doi: 10.1242/jcs.167726. Epub 2015 Apr 23. J Cell Sci. 2015. PMID: 25908855 Free PMC article. - Neuronal functions of adaptor complexes involved in protein sorting.
Guardia CM, De Pace R, Mattera R, Bonifacino JS. Guardia CM, et al. Curr Opin Neurobiol. 2018 Aug;51:103-110. doi: 10.1016/j.conb.2018.02.021. Epub 2018 Mar 17. Curr Opin Neurobiol. 2018. PMID: 29558740 Free PMC article. Review. - A Ca2+ channel differentially regulates Clathrin-mediated and activity-dependent bulk endocytosis.
Yao CK, Liu YT, Lee IC, Wang YT, Wu PY. Yao CK, et al. PLoS Biol. 2017 Apr 17;15(4):e2000931. doi: 10.1371/journal.pbio.2000931. eCollection 2017 Apr. PLoS Biol. 2017. PMID: 28414717 Free PMC article. - Exocytosis and endocytosis: modes, functions, and coupling mechanisms.
Wu LG, Hamid E, Shin W, Chiang HC. Wu LG, et al. Annu Rev Physiol. 2014;76:301-31. doi: 10.1146/annurev-physiol-021113-170305. Epub 2013 Nov 20. Annu Rev Physiol. 2014. PMID: 24274740 Free PMC article. Review.
References
- Dittman J., Ryan T.A. Molecular circuitry of endocytosis at nerve terminals. Annu. Rev. Cell Dev. Biol. 2009;25:133–160. - PubMed
- Granseth B., Odermatt B., Royle S.J., Lagnado L. Clathrin-mediated endocytosis is the dominant mechanism of vesicle retrieval at hippocampal synapses. Neuron. 2006;51:773–786. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials