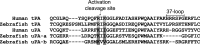Urokinase-type plasminogen activator-like proteases in teleosts lack genuine receptor-binding epidermal growth factor-like domains - PubMed (original) (raw)
. 2012 Aug 10;287(33):27526-36.
doi: 10.1074/jbc.M112.369207. Epub 2012 Jun 25.
Thomas K Kristensen, Jan K Jensen, Agnieszka Szczur, Anni Christensen, Lisbeth M Andersen, Jesper S Johansen, Niels Larsen, Erik Baatrup, Mingdong Huang, Michael Ploug, Peter A Andreasen
Affiliations
- PMID: 22733817
- PMCID: PMC3431643
- DOI: 10.1074/jbc.M112.369207
Urokinase-type plasminogen activator-like proteases in teleosts lack genuine receptor-binding epidermal growth factor-like domains
René Bager et al. J Biol Chem. 2012.
Abstract
Plasminogen activation catalyzed by urokinase-type plasminogen activator (uPA) plays an important role in normal and pathological tissue remodeling processes. Since its discovery in the mid-1980s, the cell membrane-anchored urokinase-type plasminogen activator receptor (uPAR) has been believed to be central to the functions of uPA, as uPA-catalyzed plasminogen activation activity appeared to be confined to cell surfaces through the binding of uPA to uPAR. However, a functional uPAR has so far only been identified in mammals. We have now cloned, recombinantly produced, and characterized two zebrafish proteases, zfuPA-a and zfuPA-b, which by several criteria are the fish orthologs of mammalian uPA. Thus, both proteases catalyze the activation of fish plasminogen efficiently and both proteases are inhibited rapidly by plasminogen activator inhibitor-1 (PAI-1). But zfuPA-a differs from mammalian uPA by lacking the exon encoding the uPAR-binding epidermal growth factor-like domain; zfuPA-b differs from mammalian uPA by lacking two cysteines of the epidermal growth factor-like domain and a uPAR-binding sequence comparable with that found in mammalian uPA. Accordingly, no zfuPA-b binding activity could be found in fish white blood cells or fish cell lines. We therefore propose that the current consensus of uPA-catalyzed plasminogen activation taking place on cell surfaces, derived from observations with mammals, is too narrow. Fish uPAs appear incapable of receptor binding in the manner known from mammals and uPA-catalyzed plasminogen activation in fish may occur mainly in solution. Studies with nonmammalian vertebrate species are needed to obtain a comprehensive understanding of the mechanism of plasminogen activation.
Figures
FIGURE 1.
Exon-intron structure of the genes for human uPA, zfuPA-a, and zfuPA-b. The exons of human uPA are colored alternating black and blue. The corresponding exons of the zfuPA-a and zfuPA-b are colored likewise. Extra exons in the zfuPA-a and zfuPA-b are colored green. Red indicates residues encoded across splice sites. The degree of sequence conservation is indicated by asterisks (fully conserved), colons (strongly conserved), and full stops (weak). Colored bars indicate the domains of human uPA. Potential _N_-linked glycosylation sites are underlined.
FIGURE 2.
Comparison of zebrafish and human uPAs and tPAs. The N-terminal part of the catalytic domains from uPAs and tPAs are aligned. The activation cleavage site and the 37-loop are labeled. Differences between the activation cleavage sites of tPAs and uPAs are highlighted with different shades of gray.
FIGURE 3.
Levels of zfuPA-a and zfuPA-b mRNAs in zebrafish tissue. Relative mRNA levels were determined by qPCR and normalized to the _ef1_α mRNA levels. Each data point represents mean ± S.D. values for tissues from six individuals. For each gene, data were normalized to the level in testes. Values on the y axes are arbitrary units corresponding to a standard curve of diluted mRNA and the two graphs are thus not directly comparable. A, expression levels of zfplau-a (the gene encoding zfuPA-a). B, expression levels of zfplau-b (the gene encoding zfuPA-b).
FIGURE 4.
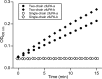
Activation of human or salmon plasminogen by human uPA or zfuPA-a or zfuPA-b. Salmon or human plasminogen (1 μ
m
) was incubated with human uPA or zfuPA-a or zfuPA-b (100 n
m
) at 37 °C. At the indicated time points, 30-μl samples were taken for SDS-PAGE under reducing conditions. After electrophoresis, the gels were stained with Coomassie Blue and dried. The relative intensities of the plasminogen, plasmin heavy chain, and plasmin light chain were determined by densitometric scanning. The fraction of the added plasminogen, which had been converted plasmin, was calculated and plotted versus the incubation time (closed squares, fish plasmin; closed triangles, human plasmin). Error bars indicate S.D. calculated from three independent experiments. A, activation by zfuPA-a. B, activation by zfuPA-b. C, activation by human uPA. D, typical SDS-PAGE showing the activation of fish plasminogen by zfuPA-a (MW, molecular weight marker; C, control without addition of zfuPA-a).
FIGURE 5.
Assay of the relative activities of the single-chain forms and the two-chain forms of zfuPA-a and zfuPA-b. Four n
m
enzyme was incubated with 100 μ
m
of the chromogenic substrate S-2288. The activities of two-chain zfuPA-a (closed circles), two-chain zfuPA-b (closed diamonds), single-chain zfuPA-a (open circles), and single-chain zfuPA-b (open diamonds) are shown. _A_405 nm, optical density at 405 nm.
FIGURE 6.
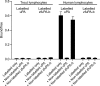
Assay of binding of human or fish uPA to human or fish lymphocytes. The binding of 125I-labeled uPA or 125I-labeled zfuPA-b to lymphocytes isolated from human blood or rainbow trout blood was estimated by incubating cells with 5 p
m
labeled uPA, in the presence or absence of 200 n
m
nonlabeled human uPA or nonlabeled zfuPA-b. The ratio between bound and free labeled ligand is reported. Error bars represents S.D. for 3 independent determinations.
FIGURE 7.
Alignment of uPA EGF-like domains from selected amniotes and fishes and other EGF-like domains. Cysteines are highlighted in gray. Residues important for the binding between human uPA and human uPAR are indicated by arrows.
Similar articles
- Expressions of urokinase-type plasminogen activator, its receptor and plasminogen activator inhibitor-1 in gastric cancer cells and effects of Helicobacter pylori.
Iwamoto J, Mizokami Y, Takahashi K, Nakajima K, Ohtsubo T, Miura S, Narasaka T, Takeyama H, Omata T, Shimokobe K, Ito M, Takehara H, Matsuoka T. Iwamoto J, et al. Scand J Gastroenterol. 2005 Jul;40(7):783-93. doi: 10.1080/00365520510015665. Scand J Gastroenterol. 2005. PMID: 16109653 - A hybrid protein of urokinase growth-factor domain and plasminogen-activator inhibitor type 2 inhibits urokinase activity and binds to the urokinase receptor.
Ballance DJ, Marshall JM, Cottingham IR, Steven J, Berry SJ, Cederholm-Williams SA, Goodey AR, Courtney M. Ballance DJ, et al. Eur J Biochem. 1992 Jul 1;207(1):177-83. doi: 10.1111/j.1432-1033.1992.tb17035.x. Eur J Biochem. 1992. PMID: 1321039 - Expression of urokinase-type plasminogen activator (uPA), its receptor (uPAR), and inhibitor (PAI-1) in human breast carcinomas and their clinical relevance.
Andres SA, Edwards AB, Wittliff JL. Andres SA, et al. J Clin Lab Anal. 2012 Feb;26(2):93-103. doi: 10.1002/jcla.21488. J Clin Lab Anal. 2012. PMID: 22467324 Free PMC article. - The urokinase plasminogen activation system in gastroesophageal cancer: A systematic review and meta-analysis.
Brungs D, Chen J, Aghmesheh M, Vine KL, Becker TM, Carolan MG, Ranson M. Brungs D, et al. Oncotarget. 2017 Apr 4;8(14):23099-23109. doi: 10.18632/oncotarget.15485. Oncotarget. 2017. PMID: 28416743 Free PMC article. Review. - Structure-function relationships in the interaction between the urokinase-type plasminogen activator and its receptor.
Ploug M. Ploug M. Curr Pharm Des. 2003;9(19):1499-528. doi: 10.2174/1381612033454630. Curr Pharm Des. 2003. PMID: 12871065 Review.
Cited by
- Identification of de novo EP300 and PLAU variants in a patient with Rubinstein-Taybi syndrome-related arterial vasculopathy and skeletal anomaly.
Park JE, Kim E, Lee DW, Park TK, Kim MS, Jang SY, Ahn J, Park KB, Kim KH, Park HC, Ki CS, Kim DK. Park JE, et al. Sci Rep. 2021 Aug 5;11(1):15931. doi: 10.1038/s41598-021-95133-0. Sci Rep. 2021. PMID: 34354133 Free PMC article. - Origin and diversification of the plasminogen activation system among chordates.
Chana-Muñoz A, Jendroszek A, Sønnichsen M, Wang T, Ploug M, Jensen JK, Andreasen PA, Bendixen C, Panitz F. Chana-Muñoz A, et al. BMC Evol Biol. 2019 Jan 17;19(1):27. doi: 10.1186/s12862-019-1353-z. BMC Evol Biol. 2019. PMID: 30654737 Free PMC article. - Targeting the Urokinase-Type Plasminogen Activator Receptor (uPAR) in Human Diseases With a View to Non-invasive Imaging and Therapeutic Intervention.
Leth JM, Ploug M. Leth JM, et al. Front Cell Dev Biol. 2021 Aug 20;9:732015. doi: 10.3389/fcell.2021.732015. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34490277 Free PMC article. Review. - Biochemical and structural analyses suggest that plasminogen activators coevolved with their cognate protein substrates and inhibitors.
Jendroszek A, Madsen JB, Chana-Muñoz A, Dupont DM, Christensen A, Panitz F, Füchtbauer EM, Lovell SC, Jensen JK. Jendroszek A, et al. J Biol Chem. 2019 Mar 8;294(10):3794-3805. doi: 10.1074/jbc.RA118.005419. Epub 2019 Jan 16. J Biol Chem. 2019. PMID: 30651349 Free PMC article.
References
- Collen D. (1999) The plasminogen (fibrinolytic) system. Thromb. Haemost. 82, 259–270 - PubMed
- Andreasen P. A., Kjøller L., Christensen L., Duffy M. J. (1997) The urokinase-type plasminogen activator system in cancer metastasis. A review. Int. J. Cancer 72, 1–22 - PubMed
- Kjaergaard M., Hansen L.V., Jacobsen B., Gardsvoll H., Ploug M. (2008) Structure and ligand interactions of the urokinase receptor (uPAR). Front. Biosci. 13, 5441–5461 - PubMed
- Dupont D. M., Madsen J. B., Kristensen T., Bodker J.S., Blouse G. E., Wind T., Andreasen P. A. (2009) Biochemical properties of plasminogen activator inhibitor-1. Front. Biosci. 14, 1337–1361 - PubMed
- Gliemann J., Nykjaer A., Petersen C. M., Jørgensen K. E., Nielsen M., Andreasen P. A., Christensen E. I., Lookene A., Olivecrona G., Moestrup S. K. (1994) The multiligand α2-macroglobulin receptor/low density lipoprotein receptor-related protein (α2MR/LRP). Ann. N.Y. Acad. Sci. 737, 20–38 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous