Pathogenic consequences of vaginal infection with CCR5-tropic simian-human immunodeficiency virus SHIVSF162P3N - PubMed (original) (raw)
Pathogenic consequences of vaginal infection with CCR5-tropic simian-human immunodeficiency virus SHIVSF162P3N
Madina Shakirzyanova et al. J Virol. 2012 Sep.
Abstract
We previously reported efficient transmission of the pathogenic R5 simian-human immunodeficiency virus SHIV(SF162P3N) isolate in Indian rhesus macaques by intravenous and intrarectal inoculations, with a switch to CXCR4 coreceptor usage in ∼50% of infected animals that progressed rapidly to disease. Since women continue to be disproportionately affected by HIV, we developed an animal model based on the intravaginal challenge of female rhesus monkeys with SHIV(SF162P3N) and sought to validate the utility of this model to study relevant aspects of HIV transmission and pathogenesis. The effect of viral dose on infection outcome was evaluated to determine the optimal conditions for the evaluation of HIV-1 preventive and therapeutic strategies. We found that the virus can successfully cross the vaginal mucosal surface to establish infection and induce disease with coreceptor switch, but with lower efficiencies compared to intravenous and rectal transmissions. In contrast to intrarectal infection, peak and cumulative viral load over a 1 year-infection period were significantly greater in macaques exposed intravaginally to lower rather than higher inoculum doses. Moreover, low and transient viremia was observed only in macaques that were challenged intravaginally twice within the same day with a high dose of virus, which can be seen as doubling the dose. Taken together, these results show that SHIV(SF162P3N) can successfully transmit across the genital mucosa, undergo coreceptor switch, and induce disease. However, the administered dose appears to impact SHIV(SF162P3N) vaginal infection outcome in an unexpected manner.
Figures
Fig 1
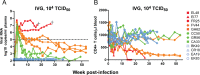
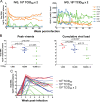
Virologic (A) and immunologic (B) measurements in RMs (n = 12) receiving a single i.v.g. inoculum of 104 TCID50 R5 SHIVSF162P3N. A “+” sign indicates euthanasia with clinical symptoms of AIDS, open symbols denote those animals that resisted challenge, and the dotted line in panel A marks a set point of 5 log10 RNA copies/ml of plasma.
Fig 2
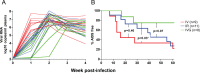
Kinetics of early virus dissemination (A) and disease-free survival curves (B) for macaques infected i.v. (n = 9), i.r. (n = 11), or i.v.g. (n = 8) with a single high dose of R5 SHIVSF162P3N. Plasma RNA levels within the first 4 weeks of infection (A) and AIDS development over a 1-year infection period (B) are shown. A P value of <0.05 is considered statistically significant.
Fig 3
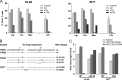
Tissue CD4+ T cell frequency (A), V3 loop sequence (B), and coreceptor usage (C) of viral variants in macaques EL48 and EI77. (A) Percentages of CD4+ T cells in the inguinal (Ing), colonic (Col), and mesenteric (Mes) lymph nodes and lamina propria lymphocyte (LPL) from the jejunum during peak (w2) and chronic (w12) stage of infection and at time of necropsy (N) are reported. Baseline values generated from three uninfected macaques (control) are shown for reference. NA, not available. (B) V3 loop sequence comparison of representative SHIVSF162P3N clones (P3N1 and P3N2) and plasma viruses in macaques EL48 and EI77 at time of necropsy. Dots indicate gaps, and dashes stand for identity in sequences, with the net positive charge of the V3 region shown on the right. Positions 11 and 25 within the V3 loop are indicated by arrows, and the 4-amino-acid deletion is underlined. The numbers in parentheses represent the numbers of clones matching the indicated sequence per total number of clones sequenced. (C) Relative entry of pseudoviruses bearing EL48 and EI77 Envs into TZM-bl, U87.CD4.CCR5, and U87.CD4.CXCR4 indicator cells. RLU, relative light units. The data are means and standard deviations from triplicate wells and are representative of at least two independent experiments.
Fig 4
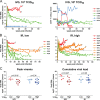
Comparison of virologic and immunologic measurements in RMs inoculated i.v.g. or i.r. with various doses R5 SHIVSF162P3N. (A) Viral load and peripheral CD4+ T cell counts in macaques receiving a single low intravaginal inoculum of 103 TCID50 R5 SHIVSF162P3N. A “+” indicates death due to euthanasia, and open symbols designate those macaques that resisted challenge. (B) Viral load in macaques inoculated i.r. with a single low (102 to 103 TCID50) or high (104 TCID50) dose of R5 SHIVSF162P3N. A “+” indicates euthanasia with clinical symptoms of AIDS. Dotted line in panels A and B mark a set-point of 5 log10 RNA copies/ml of plasma. (C) Peak and cumulative viral load (area under the curve over a 1-year infection period) comparison of R5 SHIVSF162P3N i.v.g.- and i.r.-infected macaques. The line represents the median viral RNA copies for each group. An asterisk (*) indicates statistical significance (P < 0.05).
Fig 5
(A) Virologic and immunologic measurements in RMs (n = 7) receiving two doses of 104 TCID50 R5 SHIVSF162P3N administered i.v.g. twice within the day. A “+” indicates euthanasia with clinical symptoms of AIDS, open symbols designate the macaques that resisted challenge, and dotted line marks a set-point of 5 log10 RNA copies/ml of plasma. (B) Peak viremia and cumulative viral load comparison of macaques infected i.v.g. with various doses. The line represents the median viral RNA copies for each group. An asterisk (*) indicates statistical significance (P < 0.05). (C) Virus dissemination in macaques infected i.v.g. with different doses of SHIVSF162P3N. Plasma RNA levels within the first 8 weeks of infection are shown.
Fig 6
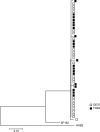
Early replicating viruses in viremic (FH84) and aviremic (DE37) R5 SHIVSF162P3N-infected macaques. A phylogenetic tree shows the relationship between Env variant sequences (V3 to V5) in plasma of macaques FH84 and DE37 at the first viral RNA-positive time point (2 wpi). A neighbor-joining tree rooted on the sequences of HxB2 and SF162 was generated. The scale bar indicates the genetic distance along the branches in nucleotides.
Similar articles
- Identification of interdependent variables that influence coreceptor switch in R5 SHIV(SF162P3N)-infected macaques.
Zhuang K, Finzi A, Toma J, Frantzell A, Huang W, Sodroski J, Cheng-Mayer C. Zhuang K, et al. Retrovirology. 2012 Dec 13;9:106. doi: 10.1186/1742-4690-9-106. Retrovirology. 2012. PMID: 23237529 Free PMC article. - Mucosal transmissibility, disease induction and coreceptor switching of R5 SHIVSF162P3N molecular clones in rhesus macaques.
Ren W, Mumbauer A, Zhuang K, Harbison C, Knight H, Westmoreland S, Gettie A, Blanchard J, Cheng-Mayer C. Ren W, et al. Retrovirology. 2013 Jan 31;10:9. doi: 10.1186/1742-4690-10-9. Retrovirology. 2013. PMID: 23369442 Free PMC article. - Efficient mucosal transmissibility but limited pathogenicity of R5 SHIV SF162P3N in Chinese-origin rhesus macaques.
Mumbauer A, Gettie A, Blanchard J, Cheng-Mayer C. Mumbauer A, et al. J Acquir Immune Defic Syndr. 2013 Apr 15;62(5):496-504. doi: 10.1097/QAI.0b013e31827f1c11. J Acquir Immune Defic Syndr. 2013. PMID: 23221980 Free PMC article. - Coreceptor switch in infection of nonhuman primates.
Cheng-Mayer C, Tasca S, Ho SH. Cheng-Mayer C, et al. Curr HIV Res. 2009 Jan;7(1):30-8. doi: 10.2174/157016209787048500. Curr HIV Res. 2009. PMID: 19149552 Review. - Minimally Modified HIV-1 Infection of Macaques: Development, Utility, and Limitations of Current Models.
Sharma M, Nag M, Del Prete GQ. Sharma M, et al. Viruses. 2024 Oct 16;16(10):1618. doi: 10.3390/v16101618. Viruses. 2024. PMID: 39459950 Free PMC article. Review.
Cited by
- The initial interplay between HIV and mucosal innate immunity.
Caputo V, Libera M, Sisti S, Giuliani B, Diotti RA, Criscuolo E. Caputo V, et al. Front Immunol. 2023 Jan 30;14:1104423. doi: 10.3389/fimmu.2023.1104423. eCollection 2023. Front Immunol. 2023. PMID: 36798134 Free PMC article. Review. - Identification of interdependent variables that influence coreceptor switch in R5 SHIV(SF162P3N)-infected macaques.
Zhuang K, Finzi A, Toma J, Frantzell A, Huang W, Sodroski J, Cheng-Mayer C. Zhuang K, et al. Retrovirology. 2012 Dec 13;9:106. doi: 10.1186/1742-4690-9-106. Retrovirology. 2012. PMID: 23237529 Free PMC article. - The number and genetic relatedness of transmitted/founder virus impact clinical outcome in vaginal R5 SHIVSF162P3N infection.
Tsai L, Tasovski I, Leda AR, Chin MP, Cheng-Mayer C. Tsai L, et al. Retrovirology. 2014 Mar 11;11:22. doi: 10.1186/1742-4690-11-22. Retrovirology. 2014. PMID: 24612462 Free PMC article. - Gp120 V5 Is Targeted by the First Wave of Sequential Neutralizing Antibodies in SHIVSF162P3N-Infected Rhesus Macaques.
Jia M, Lu H, Kong XP, Cheng-Mayer C, Wu X. Jia M, et al. Viruses. 2018 May 16;10(5):262. doi: 10.3390/v10050262. Viruses. 2018. PMID: 29772652 Free PMC article. - Epigallocatechin Gallate Inhibits Macaque SEVI-Mediated Enhancement of SIV or SHIV Infection.
Zhou RH, Guo L, Liu JB, Liu H, Hou W, Ma TC, Wang X, Wu JG, Ye L, Ho WZ, Li JL. Zhou RH, et al. J Acquir Immune Defic Syndr. 2017 Jun 1;75(2):232-240. doi: 10.1097/QAI.0000000000001361. J Acquir Immune Defic Syndr. 2017. PMID: 28328549 Free PMC article.
References
- Benki S, McClelland RS, Overbaugh J. 2005. Risk factors for human immunodeficiency virus type-1 acquisition in women in Africa. J. Neurovirol. 11(Suppl 1):58–65 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R56 AI084765/AI/NIAID NIH HHS/United States
- R01 AI084765/AI/NIAID NIH HHS/United States
- P51 OD011104/OD/NIH HHS/United States
- P51-OD011104-51/OD/NIH HHS/United States
- L60 MD003100/MD/NIMHD NIH HHS/United States
- R01AI046980/AI/NIAID NIH HHS/United States
- AI084765/AI/NIAID NIH HHS/United States
- R01 AI046980/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical