NAC functions as a modulator of SRP during the early steps of protein targeting to the endoplasmic reticulum - PubMed (original) (raw)
NAC functions as a modulator of SRP during the early steps of protein targeting to the endoplasmic reticulum
Ying Zhang et al. Mol Biol Cell. 2012 Aug.
Abstract
Nascent polypeptide-associated complex (NAC) was initially found to bind to any segment of the nascent chain except signal sequences. In this way, NAC is believed to prevent mistargeting due to binding of signal recognition particle (SRP) to signalless ribosome nascent chain complexes (RNCs). Here we revisit the interplay between NAC and SRP. NAC does not affect SRP function with respect to signalless RNCs; however, NAC does affect SRP function with respect to RNCs targeted to the endoplasmic reticulum (ER). First, early recruitment of SRP to RNCs containing a signal sequence within the ribosomal tunnel is NAC dependent. Second, NAC is able to directly and tightly bind to nascent signal sequences. Third, SRP initially displaces NAC from RNCs; however, when the signal sequence emerges further, trimeric NAC·RNC·SRP complexes form. Fourth, upon docking to the ER membrane NAC remains bound to RNCs, allowing NAC to shield cytosolically exposed nascent chain domains not only before but also during cotranslational translocation. The combined data indicate a functional interplay between NAC and SRP on ER-targeted RNCs, which is based on the ability of the two complexes to bind simultaneously to distinct segments of a single nascent chain.
Figures
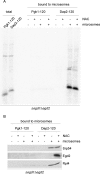
FIGURE 1:
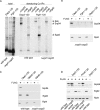
SRP does not bind to signalless RNCs in the absence of NAC. Chemical cross-linking with BS3 was performed with untagged, 35S-labeled nascent chains. FLAG–nascent chain pull downs were performed with RNCs carrying FLAG-tagged (FLAG +) or untagged (FLAG –) nascent chains generated in the absence of35S and were analyzed for the relative amount of RNCs (Rpl4), SRP (Srp54), and NAC (Egd2) via immunoblotting. To allow for a direct comparison of RNC occupation with NAC or SRP, similar amounts of RNCs (Rpl4) were loaded to a single gel. In each experiment Dap2-120-RNCs served as a control for SRP and NAC binding. For details on the nascent chains and methods see Supplemental Figures S2 and S3. (A) Chemical cross-linking of RNCs carrying 120-residue Dap2 or Pgk1 generated in a wild-type or Δ_egd1_Δ_egd2_ translation extract. Aliquots of the total after BS3 cross-linking were applied to immunoprecipitation reactions under denaturing conditions using antibodies as indicated. Samples were run on Tris-tricine gels and were analyzed via autoradiography. The total represents 5% of the amount added to each immunoprecipitation reaction. The size of the cross-links (xl) between the nascent chains and Srp54, Ssb, or Egd2, respectively, is indicated at the right. (B) FLAG–nascent chain pull down of RNCs carrying 120-residue Dap2, Pgk1, or Dap2α generated in a Δ_egd1_Δ_egd2_ translation extract. (C) FLAG–nascent chain pull down of RNCs carrying 120-residue Dap2 or OM45 generated in a wild-type or Δ_egd1_Δ_egd2_ translation extract as indicated. (D) FLAG–nascent chain pull down of RNCs carrying 120-residue Dap2 or Pho8 generated in a wild-type, Δ_egd1_Δ_egd2_, or Δ_srp54_ translation extract.
FIGURE 2:
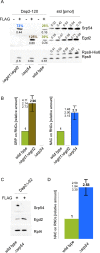
NAC and SRP compete for RNCs, exposing SA segments. FLAG–nascent chain pull downs were performed as described in Figure 1 with RNCs carrying FLAG-tagged (FLAG +) or untagged (FLAG –) nascent chains. (A) FLAG–nascent chain pull downs of RNCs carrying 120-residue Dap2 generated in wild-type, Δ_srp54_, or Δ_egd1_Δ_egd2_ translation extract. Increasing amounts of purified Rps9a-His6 (Rps9-His6), His6-Srp54 (Srp54), and NAC (Egd2) were used as standard. The amount of standard proteins loaded is given in picomoles above the protein bands. Black numbers below the protein bands indicate the amount of NAC, SRP, and RNCs in picomoles as determined based on calibration curves derived from the standard proteins. Colored numbers above the protein bands indicate the calculated percentage of RNCs occupied by NAC or SRP, respectively. (B) Relative occupation of RNCs exposing the SA segment far outside of the ribosomal tunnel. Experiments were performed as in A. Occupation of Dap2-120-RNCs generated in a wild-type translation extract was set to 1. Occupation of Dap2-120-RNCs generated in Δ_srp54_ or Δ_egd1_Δ_egd2_ translation extract is given relative to the occupation of wild-type RNCs (Materials and Methods). Error bars indicate the SE of three independent experiments; the actual individual results are indicated to the left of the error bar. (C) FLAG–nascent chain pull downs of RNCs carrying 62-residue Dap2Δ (Supplemental Figure S2) generated in wild-type or Δ_srp54_ translation extract. (D) Relative occupation of Dap2Δ-62-RNCs exposing the SA segment adjacent to the ribosomal tunnel. Analysis was performed as described in B. Error bars indicate the SE of three independent experiments; the actual individual results are indicated to the left of the error bar.
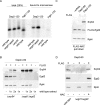
FIGURE 3:
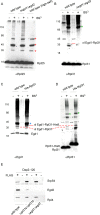
Egd1 forms a cross-link with Rpl31 but does not from a cross-link with Rpl25. Cross-linking was performed using ribosomes isolated under low-salt conditions or, if indicated, under high-salt conditions. Ribosome preparations generated from wild type, the Δ_egd1_Δ_egd2_, or the His-Rpl31 strain were incubated in either the presence (+) or the absence (–) of BS3. The molecular masses of the proteins of interest are Rpl25 (16 kDa), Rpl31 (13 kDa), Egd1 (17 kDa), and Egd2 (19 kDa). FLAG–nascent chain pull downs were performed as described in Figure 1. (A) Aliquots of a cross-linking reaction were analyzed using an antibody directed against Rpl25 (αRpl25). Cross-link products observed after high-salt treatment (labeled with red asterisks) are most likely to be unidentified ribosomal neighbors of Rpl25. (B) Aliquots were analyzed with an antibody directed against Rpl31 (αRpl31). The prominent cross-link between Rpl31 and Zuo1 (Peisker et al., 2008) is labeled with a green asterisk. The cross-link between Egd1 and Rpl31 (xl Egd1-Rpl31) is labeled with a red bar. (C) Aliquots were analyzed with an antibody recognizing the Egd1 subunit of NAC (αEgd1). The prominent cross-link between Egd1 and Egd2 is labeled with a blue asterisk. The cross-link between wild-type Rpl31 and Egd1 (xl Egd1-Rpl31) and the size-shifted cross-link between Rpl31-His8 and Egd1 (xl Egd1-Rpl31-His8) are indicated with red bars. (D) Aliquots as shown in C were analyzed using antibodies directed against Rpl31 (αRpl31). The cross-link between wild-type Rpl31 and Egd1 (xl Egd1-Rpl31) and the size-shifted cross-link between Rpl31-His8 and Egd1 (xl Egd1-Rpl31-His8), which are detected with αRpl31, comigrated with the cross-links recognized by αEgd1 shown in C. The cross-link between Rpl31 and Zuo1 and the size-shifted cross-link between Rpl31-His8 and Zuo1 are labeled with green asterisks. Because the strong cross-link product between Zuo1 and Rpl31 is otherwise overexposed, a shorter exposure of the same immunoblot is shown for the upper part. (E) FLAG–nascent chain pull downs of RNCs carrying 120-residue Dap2 generated in wild-type, Δ_rpl31a_Δ_rpl31b_, or Δ_egd1_Δ_egd2_ translation extract were performed as described in Figure 1.
FIGURE 4:
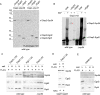
NAC and SRP can bind to the same RNC molecule if the SA segment is positioned far outside of the ribosomal tunnel. (A) RNCs carrying untagged (FLAG –) nascent chains were generated in a FLAG-Egd2 translation extract. A mock translation reaction lacking mRNA (– mRNA) was performed in the FLAG-Egd2 translation extract as a control. FLAG-NAC pull downs (Supplemental Figure S3) were then performed to isolate ribosomes and RNCs bound to FLAG-NAC. The isolated material was analyzed via immunoblots using αSrp54, αEgd2 to detect FLAG-NAC (Egd2-FLAG), and αRpl4 to detect ribosomes and RNCs. (B) As in A, using FLAG-NAC RNCs carrying nascent Dap2 of increasing length as indicated (Supplemental Figure S2). A translation reaction lacking mRNA (– mRNA) and wild-type RNCs carrying Dap2-120 were used as controls. (C) RNCs carrying FLAG-tagged (FLAG +) or untagged (FLAG –) nascent chains were generated in a translation extract derived from wild type or the Δ_egd1_Δ_egd2_ strain. If indicated, after cycloheximide addition, purified NAC (NAC +) was added to a final concentration of 1 μM and was allowed to bind to ribosomes and RNCs for 10 min at 20°C. Subsequently, FLAG–nascent chain pull downs (Supplemental Figure S3) were performed as described in Figure 1. Relative occupation of RNCs with SRP (SRP on RNCs) was calculated as described in Materials and Methods. The occupation of Dap2-120-RNCs generated in a wild-type translation extract was set to 1.
FIGURE 5:
NAC binds to the SA segment directly and in a salt-resistant manner. (A) Site-specific cross-linking to 35S-labeled nascent chains (Supplemental Figure S3D). The photo probe was positioned within the SA segment at residue 39 (Dap2-stop39) or 37 (Dap2-stop37) of Dap2-120 (Supplemental Figure S2). Cross-link products were isolated via immunoprecipitation under denaturing conditions using αSrp54, αEgd2, or αEgd1 as indicated and were subsequently visualized via autoradiography. xl Dap2-Srp54, cross-link product between the nascent chain and Srp54; xl Dap2-Egd2, cross-link product between the nascent chain and Egd2; and xl Dap2-Egd1, cross-link product between the nascent chain and Egd1. (B) Chemical cross-linking (Supplemental Figure S3) of RNCs carrying radiolabeled Dap2Δ-62. RNCs were generated in either a wild-type or a Δ_srp54_ translation extract as indicated. Analysis of the cross-link products was as described in A using αSrp54 and αEgd2. The total represents 33% of the amount added to immunoprecipitation reactions. Samples of cross-link experiments were analyzed via autoradiography. The cross-link between Dap2Δ-62 and Srp54 is labeled with a red asterisk; the cross-link between Dap2Δ-62 and Egd2 is labeled with a green asterisk. (C) RNCs carrying FLAG-tagged (FLAG +) nascent chains as indicated were generated in a wild-type or Δ_srp54_ translation extract. FLAG–nascent chain pull downs were performed with RNCs isolated under low-salt (L) or high-salt (H) conditions (see Materials and Methods). The isolated material was then analyzed for the presence of SRP (Srp54), NAC (Egd2), and RNCs (Rpl4) via immunoblotting. (D) Dap2-120-RNCs were generated in a translation extract derived from the Δ_egd1_Δ_egd2_ strain. After inhibition of translation, purified NAC was added as described in Figure 4. Subsequently, RNCs were isolated under low-salt (L) or high-salt (H) conditions, and the isolated material was analyzed as in C. (E) RNCs carrying FLAG-tagged (FLAG +) or untagged (FLAG –) 62-residue nascent OM45 (Supplemental Figure S2) were generated in wild-type translation extract. FLAG–nascent chain pull downs were preformed with low-salt-treated (L) or high-salt-treated (H) RNCs and were analyzed as described.
FIGURE 6:
NAC·RNC·SRP complexes are targeted to ER membranes. RNCs were generated in Δ_egd1_Δ_egd2_ translation extract. Wild-type (+ NAC) or Δ_egd1_Δ_egd2_ (– NAC) extract was added after inhibition of translation). (A) 35S-Labeled RNCs complemented with either wild-type translation extract (+ NAC) or ∆egd1∆egd2 translation extract (– NAC) were allowed to bind to high-salt-washed microsomal membranes (+ microsomes). After reisolation, microsomal membranes and bound RNCs were run on Tris-tricine gels and were analyzed via autoradiography (Supplemental Figure S3E). The total represents 10% of Pgk1-120 or Dap2-120-RNCs added to high-salt-washed microsomes. (B) RNCs generated as in A but in the absence of 35S were allowed to bind to microsomal membranes. After reisolation of microsomal membranes, aliquots were analyzed via immunoblotting using the indicated antibodies.
FIGURE 7:
NAC is required for the recruitment of SRP when the SA segment is localized inside the ribosomal tunnel. (A) 35S-Labeled RNCs generated in wild-type, Δ_egd1_Δ_egd2_, or Δ_srp54_ translation extract were allowed to bind to high-salt-washed microsomal membranes. After reisolation, microsomal membranes and bound RNCs were run on Tris-tricine gels and were analyzed via autoradiography. The total represents 25% of Pgk1-120 or Dap2-120-RNCs added to high-salt-washed microsomes. (B) FLAG–nascent chain pull downs with RNCs carrying Dap2∆-62 (Supplemental Figure S2). RNCs were generated in Δ_egd1_Δ_egd2_ or ∆srp54 translation extract to which wild-type extract containing NAC and SRP was added after inhibition of translation. The amount of wild-type extract corresponded to 1× or 2× the volume of the Δ_egd1_Δ_egd2_ or a ∆srp54 translation extract used in the translation reaction. FLAG–nascent chain pull downs (Supplemental Figure S3B) were then performed and were analyzed as described in Figure 1. (C) RNCs carrying untagged Dap2-60 or Dap2-120 were generated in a FLAG-Egd2 translation extract. Subsequently FLAG-NAC pull downs (Supplemental Figure S3C) were performed and were analyzed as in Figure 4. (D) RNCs carrying FLAG-tagged (FLAG +) or untagged (FLAG –) Dap2-60 were generated in wild-type or Δ_egd1_Δ_egd2_ translation extract. As indicated, the Δ_egd1_Δ_egd2_ translation extract was complemented with a final concentration of 1 μM purified NAC (+ NAC) before the translation reaction.
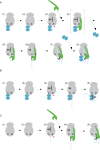
FIGURE 8:
Model of the interactions between NAC and SRP on ribosomes involved in the translation of a protein destined for cotranslational translocation to the ER. (A) Sequence of events in the presence of NAC and SRP. 1) NAC binds to empty ribosomes and 2) remains bound when translation initiates. 3) NAC·RNC complexes remain stable when a SA segment emerges inside the ribosomal tunnel. The SA segment may induce conformational changes in the SRP binding site. 4) SRP is recruited, and NAC is concomitantly released. At this stage trimeric complexes are unstable. This is likely due to sterical hindrance between domains of NAC and SRP, which are not directly involved in ribosome binding. 5, 6) When the SA segment emerges SRP binds to it directly and in a salt-resistant manner. At this stage NAC and SRP cannot bind simultaneously to an RNC. 7, 8) On further elongation of the nascent chain, segments C-terminal of the SA exit the tunnel. Now NAC can rebind, and trimeric NAC·RNC·SRP complexes form, which can interact with the translocation machinery in the ER membrane. (B) Sequence of events when SRP is absent or SRP binding is delayed. 1–3) as in A. 4,5) Because SRP is absent, NAC·RNC complexes remain stable, and NAC establishes salt-resistant interactions with the nascent SA segment. If added to the system at stage 4, SRP displaces NAC. This order of events allows NAC to shield a SA segment until SRP is available. (C) Sequence of events when NAC is absent. 1, 2) SRP binds poorly to nontranslating ribosomes or signalless RNCs. 3) SRP is not recruited to RNCs containing a SA segment inside the ribosomal tunnel. 4, 5) As soon as the SA segment emerges outside of the tunnel, SRP binds to the RNC and the SA segment in a salt-resistant manner. If added to the system at stage 4, NAC is unable to displace SRP. For details see Results and Discussion. The ribosome is shown in gray, NAC is shown in blue, and SRP is shown in green. The nascent chain is depicted in light blue, and the SA segment is indicated in black. The red arrow points to the SRP-binding site. The major ribosomal binding sites of SRP (Rpl25) and NAC (Rpl31) are indicated.
Similar articles
- Binding of signal recognition particle gives ribosome/nascent chain complexes a competitive advantage in endoplasmic reticulum membrane interaction.
Neuhof A, Rolls MM, Jungnickel B, Kalies KU, Rapoport TA. Neuhof A, et al. Mol Biol Cell. 1998 Jan;9(1):103-15. doi: 10.1091/mbc.9.1.103. Mol Biol Cell. 1998. PMID: 9436994 Free PMC article. - The intrinsic ability of ribosomes to bind to endoplasmic reticulum membranes is regulated by signal recognition particle and nascent-polypeptide-associated complex.
Lauring B, Kreibich G, Weidmann M. Lauring B, et al. Proc Natl Acad Sci U S A. 1995 Oct 10;92(21):9435-9. doi: 10.1073/pnas.92.21.9435. Proc Natl Acad Sci U S A. 1995. PMID: 7568149 Free PMC article. - A signal-anchor sequence stimulates signal recognition particle binding to ribosomes from inside the exit tunnel.
Berndt U, Oellerer S, Zhang Y, Johnson AE, Rospert S. Berndt U, et al. Proc Natl Acad Sci U S A. 2009 Feb 3;106(5):1398-403. doi: 10.1073/pnas.0808584106. Epub 2009 Jan 21. Proc Natl Acad Sci U S A. 2009. PMID: 19164516 Free PMC article. - Fidelity of Cotranslational Protein Targeting to the Endoplasmic Reticulum.
Hsieh HH, Shan SO. Hsieh HH, et al. Int J Mol Sci. 2021 Dec 28;23(1):281. doi: 10.3390/ijms23010281. Int J Mol Sci. 2021. PMID: 35008707 Free PMC article. Review. - Co-translational targeting and translocation of proteins to the endoplasmic reticulum.
Nyathi Y, Wilkinson BM, Pool MR. Nyathi Y, et al. Biochim Biophys Acta. 2013 Nov;1833(11):2392-402. doi: 10.1016/j.bbamcr.2013.02.021. Epub 2013 Feb 26. Biochim Biophys Acta. 2013. PMID: 23481039 Review.
Cited by
- GSK-3β-dependent downregulation of γ-taxilin and αNAC merge to regulate ER stress responses.
Hotokezaka Y, Katayama I, van Leyen K, Nakamura T. Hotokezaka Y, et al. Cell Death Dis. 2015 Apr 16;6(4):e1719. doi: 10.1038/cddis.2015.90. Cell Death Dis. 2015. PMID: 25880086 Free PMC article. - Disruption of the nascent polypeptide-associated complex leads to reduced polyglutamine aggregation and toxicity.
Dublin-Ryan LB, Bhadra AK, True HL. Dublin-Ryan LB, et al. PLoS One. 2024 Aug 15;19(8):e0303008. doi: 10.1371/journal.pone.0303008. eCollection 2024. PLoS One. 2024. PMID: 39146256 Free PMC article. - Crosslinking and Reconstitution Approaches to Study Protein Transport.
Kuhn A. Kuhn A. Protein J. 2019 Jun;38(3):229-235. doi: 10.1007/s10930-019-09842-7. Protein J. 2019. PMID: 31144202 Review. - ATM-associated signalling triggers the unfolded protein response and cell death in response to stress.
Hotokezaka Y, Katayama I, Nakamura T. Hotokezaka Y, et al. Commun Biol. 2020 Jul 14;3(1):378. doi: 10.1038/s42003-020-1102-2. Commun Biol. 2020. PMID: 32665601 Free PMC article. - The nascent polypeptide-associated complex is essential for autophagic flux.
Guo B, Huang J, Wu W, Feng D, Wang X, Chen Y, Zhang H. Guo B, et al. Autophagy. 2014 Oct 1;10(10):1738-48. doi: 10.4161/auto.29638. Epub 2014 Jul 18. Autophagy. 2014. PMID: 25126725 Free PMC article.
References
- Bukau B, Deuerling E, Pfund C, Craig EA. Getting newly synthesized proteins into shape. Cell. 2000;101:119–122. - PubMed
- Cheng Z, Gilmore R. Slow translocon gating causes cytosolic exposure of transmembrane and lumenal domains during membrane protein integration. Nat Struct Mol Biol. 2006;13:930–936. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases