The neuroprotective effects of milk fat globule-EGF factor 8 against oligomeric amyloid β toxicity - PubMed (original) (raw)
The neuroprotective effects of milk fat globule-EGF factor 8 against oligomeric amyloid β toxicity
Endong Li et al. J Neuroinflammation. 2012.
Abstract
Background: Phosphatidylserine receptor is a key molecule that mediates the phagocytosis of apoptotic cells. Milk fat globule-EGF factor 8 (MFG-E8) is a phosphatidylserine receptor that is expressed on various macrophage lineage cells, including microglia in the central nervous system (CNS). Targeted clearance of degenerated neurons by microglia is essential to maintain healthy neural networks. We previously showed that the CX3C chemokine fractalkine is secreted from degenerated neurons and accelerates microglial clearance of neuronal debris via inducing the release of MFG-E8. However, the mechanisms by which microglia produce MFG-E8 and the precise functions of MFG-E8 are unknown.
Methods: The release of MFG-E8 from microglia treated with conditioned medium from neurons exposed to neurotoxic substances, glutamate or oligomeric amyloid β (oA β) was measured by ELISA. The neuroprotective effects of MFG-E8 and MFG-E8-induced microglial phagocytosis of oA β were assessed by immunocytochemistry. The effects of MFG-E8 on the production of the anti-oxidative enzyme hemeoxygenase-1 (HO-1) were determined by ELISA and immunocytochemisty.
Results: MFG-E8 was induced in microglia treated with conditioned medium from neurons that had been exposed to neurotoxicants, glutamate or oA β. MFG-E8 significantly attenuated oA β-induced neuronal cell death in a primary neuron-microglia coculture system. Microglial phagocytosis of oA β was accelerated by MFG-E8 treatment due to increased CD47 expression in the absence of neurotoxic molecule production, such as tumor necrosis factor-α, nitric oxide, and glutamate. MFG-E8-treated microglia induced nuclear factor E(2)-related factor 2 (Nrf2)-mediated HO-1 production, which also contributed to neuroprotection.
Conclusions: These results suggest that microglia release MFG-E8 in response to signals from degenerated neurons and that MFG-E8 protects oA β-induced neuronal cell death by promoting microglial phagocytic activity and activating the Nrf2-HO-1 pathway. Thus, MFG-E8 may have novel roles as a neuroprotectant in neurodegenerative conditions.
Figures
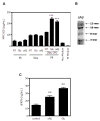
Figure 1
Induction of MFG-E8 by soluble factors released from degenerated neurons. (A) The levels of MFG-E8 secreted from microglia (Mi) and cortical neurons (Neu) treated with 20 μM glutamate (Glu) or 5 μM oligomeric amyloid β (oAβ) were measured by ELISA. In addition, MFG-E8 released from microglia exposed to neuronal conditioned medium (Neu CM) that had been treated with Glu or oAβ was also measured. Results are presented as the means with S.E.M. (n = 3). Glu- or oAβ-treated neuronal conditioned medium significantly induced MFG-E8 expression from microglia compared with the untreated control samples. ***: P <0.001 (one-way ANOVA with Dunnett’s post-hoc test). (B) The Western blot data of oAβ used in the present study. The blot was incubated in mouse anti-Aβ monoclonal antibody (6E10) (1:1,000, Chemicon). (C) The levels of the soluble secreted form of fractalkine (sFKN) released from cortical neurons treated with 20 μM glutamate (Glu) or 5 μM oligomeric amyloid β (oAβ) were measured. The results are presented as the means with S.E.M. (n = 3). Glu and oAβ treatment significantly induced sFKN release from neurons compared to the untreated control samples. **: P <0.01 (one-way ANOVA with Dunnett’s post-hoc test).
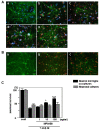
Figure 2
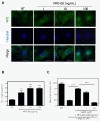
MFG-E8 exerts neuroprotective effects in the presence of microglia. The effect of MFG-E8 treatment against oAβ toxicity in both neuron − microglia co-cultures (A) and neuronal cultures (B). Neurons were stained with an anti-MAP-2 antibody (green), microglia were stained with a Cy5-conjugated anti-CD11b antibody (blue), and oAβ was stained with an anti-4 G8 antibody (red). Scale bar = 50 μm. Untreated neuronal cultures and neuron − microglia cocultures (1:2 neurons to microglia) (cont; a). Five micromolar of oAβ induced neuronal loss in both neuronal and neuron–microglia cocultures (b). In the presence of microglia, MFG-E8 treatment induced neuroprotective effects in a dose-dependent manner (doses were 1 ng/mL (c), 10 ng/mL (d), 100 ng/mL (e) in (A)). On the other hand, MFG-E8 did not show any effects on oAβ-induced neurotoxicity, even at the highest dose (100 ng/mL; c in (B)). (C) Neuronal survival was estimated as the percentage of intact neurons in the treated sample relative to the untreated sample. The results are presented as the means with S.E.M. (n = 3), in which 10 randomly selected fields were analyzed. Significant differences compared to samples only treated with oAβ are noted. *: P <0.05, ***: P <0.001, (one-way ANOVA with Dunnett’s post-hoc test).
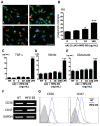
Figure 3
MFG-E8 induces microglial phagocytosis of oAβ. (A) Microglia were pre-treated with 1 ng/mL (b), 10 ng/mL (c) or 100 ng/mL (d) MFG-E8 for 1 h, and then 5 μM oAβ was added to the culture for 6 h. Primary antibodies recognizing Rab7 (green), oAβ (red) and nuclei (blue) were used (a − d). A few oAβ-incorporated microglia were detected in the absence of MFG-E8 (a), while a sufficient number of microglia that had engulfed oAβ were detected in the presence of the higher MFG-E8 dose (d) under confocal laser scanning microscope. Scale bar = 50 μm. (B) Quantification of the phagocytosis index, which is defined as the percentage of total microglial-Rab7 staining (green) that overlaps with oAβ staining (red). The results are presented as the means with S.E.M. (n = 3), in which 10 randomly selected fields were analyzed. Significant differences compared with samples that were only treated with oAβ (oAβ) are noted. *: P <0.05 (one-way ANOVA with Dunnett’s post-hoc test). The measurement of TNF-α (C), nitrite (D), and glutamate (E) produced by microglia treated with MFG-E8 plus oAβ was performed. After 24 h treatment with 1 ng/mL, 10 ng/mL or 100 ng/mL MFG-E8 with 5 μM oAβ, the supernatants of microglial cultures were analyzed. 100 ng/mL LPS treatment for 24 h was used as a positive control. The results are presented as the means with S.E.M. (n = 5). Significant differences compared to untreated microglia (NT) are noted. ***: P <0.001, (one-way ANOVA with Dunnett’s post-hoc test). (F) mRNA expression levels of oAβ phagocytosis-related cellular membrane surface antigens, CD36 and CD47, in microglia treated with 100 ng/mL MFG-E8 for 24 h was assessed using RT-PCR. GAPDH expression was used as a control. (D) CD36 and CD47 protein levels were assessed by FACS analysis. Microglia were treated with 100 ng/mL MFG-E8 for 72 h. The gray-filled curve represents the isotype-matched control. The black and blue lines indicate untreated (NT) and MFG-E8-treated samples, respectively.
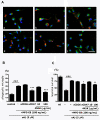
Figure 4
MFG-E8 enhances microglial phagocytosis of oAβ through CD47. (A) Microglia were treated with 1 μg/mL of isotype-matched control IgG (a, b), 100 ng/mL MFG-E8 (b − f), 1 μg/mL of anti-CD36 neutralizing antibody (c), 1 μg/mL of anti-CD47 neutralizing antibody (d), the peptide antagonist 4N1K 10 μg/mL (e) or 100 μg/mL (f) for 1 h, and then 5 μM oAβ was added to the culture for 24 h. Primary antibodies against Rab7 (green), oAβ (red) and nuclei (blue) were used (a − f). Scale bar = 50 μm. (B) Quantification of the phagocytosis index. Results show the means with S.E.M. (n = 3), in which 10 randomly selected fields were analyzed. Significant differences compared with the isotype-matched control samples (#) or samples treated only with MFG-E8 (*) are noted. ***: P <0.001; ###: P <0.001 (one-way ANOVA with Tukey’s post-hoc test). (C) The effects of blockade of CD36 and CD47 in MFG-E8-treated microglia on neuronal survival. Neuron − microglia co-cultures were pre-treated with MFG-E8 for 1 h in the presence of anti-CD36 neutralizing antibody, anti-CD47 neutralizing antibody, and 10 μg/mL or 100 μg/mL 4N1K. Then, the cultures were treated with oAβ for 24 h. The neuronal survival rate was estimated. The results are presented as the means with S.E.M. (n = 5), in which 10 randomly selected fields were analyzed. Significant differences compared with the isotype-matched control samples (#) or samples treated only with MFG-E8 (*) are noted. *: P <0.05; ###: P <0.001 (one-way ANOVA with Tukey’s post-hoc test).
Figure 5
MFG-E8 exerts neuroprotective effects by activating the Nrf-2-HO-1 pathway in microglia. (A) Immunofluorescence images of Nrf2 (green) and nuclei (Hoechst; blue) in microglia treated with the indicated concentrations of MFG-E8. Scale bar = 10 μm. (B) After microglia were treated with the indicated concentrations of MFG-E8 for 24 h, HO-1 protein expression levels were measured by ELISA. Significant differences compared with untreated samples (*) are noted. ***: P <0.001 (one-way ANOVA with Dunnett’s post-hoc test). (C) Neuron − microglia co-cultures were pre-treated with MFG-E8 for 3 h in the presence of the HO-1 inhibitor SnMP. Then, the cultures were treated with oAβ for 24 h. The neuronal survival rate in the presence of MFG-E8 and SnMP was estimated. The results are presented as the means with S.E.M. (n = 3), in which 10 randomly selected fields were analyzed. Significant differences compared with oAβ plus MFG-E8 treated samples are noted. ***: P <0.001 (one-way ANOVA with Dunnett’s post-hoc test).
Figure 6
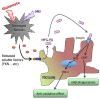
Model of the neuroprotective role of MFG-E8 against Aβ toxicity. MFG-E8 is secreted from microglia in response to soluble factors (for example, FKN) produced by degenerated neurons. MFG-E8 enhances Aβ phagocytosis by microglia through CD47 expression. MFG-E8 also directly increases the nuclear translocation of the transcriptional factor Nrf2, which induces expression of the antioxidant enzyme HO-1. Therefore, MFG-E8 is a potent downstream molecule of sFKN and is an intrinsic neuroprotectant for damaged yet surviving neurons.
References
- Noda M, Doi Y, Liang J, Kawanokuchi J, Sonobe Y, Takeuchi H, Mizuno T, Suzumura A. Fractalkine attenuates excito-neurotoxicity via microglial clearance of damaged neurons and antioxidant enzyme heme oxygenase-1 expression. J Biol Chem. 2011;286:2308–2319. doi: 10.1074/jbc.M110.169839. - DOI - PMC - PubMed
- Boddaert J, Kinugawa K, Lambert JC, Boukhtouche F, Zoll J, Merval R, Blanc-Brude O, Mann D, Berr C, Vilar J, Garabedian B, Journiac N, Charue D, Silvestre JS, Duyckaerts C, Amouyel P, Mariani J, Tedgui A, Mallat Z. Evidence of a role for lactadherin in Alzheimer's disease. Am J Pathol. 2007;170:921–929. doi: 10.2353/ajpath.2007.060664. - DOI - PMC - PubMed
- Kranich J, Krautler NJ, Falsig J, Ballmer B, Li S, Hutter G, Schwarz P, Moos R, Julius C, Miele G, Aguzzi A. Engulfment of cerebral apoptotic bodies controls the course of prion disease in a mouse strain-dependent manner. J Exp Med. 2010;207:2271–2281. doi: 10.1084/jem.20092401. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous