Exocrine ontogenies: on the development of pancreatic acinar, ductal and centroacinar cells - PubMed (original) (raw)
Review
Exocrine ontogenies: on the development of pancreatic acinar, ductal and centroacinar cells
Megan H Cleveland et al. Semin Cell Dev Biol. 2012 Aug.
Abstract
This review summarizes our current understanding of exocrine pancreas development, including the formation of acinar, ductal and centroacinar cells. We discuss the transcription factors associated with various stages of exocrine differentiation, from multipotent progenitor cells to fully differentiated acinar and ductal cells. Within the branching epithelial tree of the embryonic pancreas, this involves the progressive restriction of multipotent pancreatic progenitor cells to either a central "trunk" domain giving rise to the islet and ductal lineages, or a peripheral "tip" domain giving rise to acinar cells. This review also discusses the soluble morphogens and other signaling pathways that influence these events. Finally, we examine centroacinar cells as an enigmatic pancreatic cell type whose lineage remains uncertain, and whose possible progenitor capacities continue to be explored.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures
Figure 1. Transcription factor expression in adult ductal epithelium
Immunofluorescent labeling for Sox9, Hnf6 and Hnf1β in adult mouse pancreas. Note heterogeneous expression, with neighboring cells often displaying different patterns of transcription factor expression.
Figure 2. Transcription factor topologies in developing mouse pancreas
Immunofluorescent labeling for Pdx1, Sox9, Nkx6.1, Ptf1a, Ngn3 and E-cadherin (Ecad) in emerging trunk and tip domains of developing mouse pancreas. Hnf1β and Nkx6.1 become progressively restricted to the central trunk region of the branching pancreatic epithelium, from which Ngn+ cells begin to emerge. In contrast, Ptf1a is restricted to peripheral tips. As late as E13.5, Pdx1 and Sox9 are expressed in both central trunk and and peripheral tip domains.
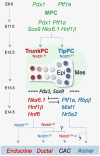
Figure 3. Emergence of ductal, islet and acinar lineages from progressively restricted trunk and tip progenitor cells
Initially tri-potent multil-ineage progenitor cells (MPCs) expressing both Pdx1 and Ptf1a ultimately give rise to ductal, islet and and acinar cells. On E11.5 most MPCs also co-express Sox9, Hnf1 β, Hnf6, Nkx6.1, and Nkx6.2. By E12.5, Hnf1 β, Hnf6, Nkx6.1, and Nkx6.2 become largely restricted to trunk progenitor cells (TrunkPCs), while Ptf1a becomes restricted to tip progenitors (TipPCs), with Notch signaling promoting the TrunkPC identity. By E13.5, acinar and lslet differentiation have been initiated, associated with the activation of additional lineage-specific transcription factors. Among TrunkPCs, Notch activation promotes a ductal fate. By the time of gestation, active Notch signaling is largely confined to centroacinar cells (CAC), whose derivation from Trunk vs. TipPCs remains unknown. CACs may be derived from either trunk progenitors maintaining active Notch signaling or from tip progenitors reactivating Notch.
Similar articles
- ptf1a+ , ela3l- cells are developmentally maintained progenitors for exocrine regeneration following extreme loss of acinar cells in zebrafish larvae.
Schmitner N, Kohno K, Meyer D. Schmitner N, et al. Dis Model Mech. 2017 Mar 1;10(3):307-321. doi: 10.1242/dmm.026633. Epub 2017 Jan 30. Dis Model Mech. 2017. PMID: 28138096 Free PMC article. - Competence of failed endocrine progenitors to give rise to acinar but not ductal cells is restricted to early pancreas development.
Beucher A, Martín M, Spenle C, Poulet M, Collin C, Gradwohl G. Beucher A, et al. Dev Biol. 2012 Jan 15;361(2):277-85. doi: 10.1016/j.ydbio.2011.10.025. Epub 2011 Oct 26. Dev Biol. 2012. PMID: 22056785 Free PMC article. - The nuclear hormone receptor family member NR5A2 controls aspects of multipotent progenitor cell formation and acinar differentiation during pancreatic organogenesis.
Hale MA, Swift GH, Hoang CQ, Deering TG, Masui T, Lee YK, Xue J, MacDonald RJ. Hale MA, et al. Development. 2014 Aug;141(16):3123-33. doi: 10.1242/dev.109405. Epub 2014 Jul 25. Development. 2014. PMID: 25063451 Free PMC article. - Lineage commitment and cellular differentiation in exocrine pancreas.
Means AL, Leach SD. Means AL, et al. Pancreatology. 2001;1(6):587-96. doi: 10.1159/000055868. Pancreatology. 2001. PMID: 12120241 Review. - Development of the Pancreatic Ducts and Their Contribution to Organogenesis.
Dale DJ, Rutan CD, Mastracci TL. Dale DJ, et al. Adv Anat Embryol Cell Biol. 2024;239:31-55. doi: 10.1007/978-3-031-62232-8_2. Adv Anat Embryol Cell Biol. 2024. PMID: 39283481 Review.
Cited by
- Defense Mechanisms Against Acid Exposure by Dental Enamel Formation, Saliva and Pancreatic Juice Production.
Racz R, Nagy A, Rakonczay Z, Dunavari EK, Gerber G, Varga G. Racz R, et al. Curr Pharm Des. 2018;24(18):2012-2022. doi: 10.2174/1381612824666180515125654. Curr Pharm Des. 2018. PMID: 29769002 Free PMC article. Review. - Exploring human pancreatic organoid modelling through single-cell RNA sequencing analysis.
Cherubini A, Rusconi F, Piras R, Wächtershäuser KN, Dossena M, Barilani M, Mei C, Hof L, Sordi V, Pampaloni F, Dolo V, Piemonti L, Lazzari L. Cherubini A, et al. Commun Biol. 2024 Nov 18;7(1):1527. doi: 10.1038/s42003-024-07193-3. Commun Biol. 2024. PMID: 39558019 Free PMC article. - Aldh1b1 expression defines progenitor cells in the adult pancreas and is required for Kras-induced pancreatic cancer.
Mameishvili E, Serafimidis I, Iwaszkiewicz S, Lesche M, Reinhardt S, Bölicke N, Büttner M, Stellas D, Papadimitropoulou A, Szabolcs M, Anastassiadis K, Dahl A, Theis F, Efstratiadis A, Gavalas A. Mameishvili E, et al. Proc Natl Acad Sci U S A. 2019 Oct 8;116(41):20679-20688. doi: 10.1073/pnas.1901075116. Epub 2019 Sep 23. Proc Natl Acad Sci U S A. 2019. PMID: 31548432 Free PMC article. - Changes in Pancreatic Senescence Mediate Pancreatic Diseases.
Li K, Bian J, Xiao Y, Wang D, Han L, He C, Gong L, Wang M. Li K, et al. Int J Mol Sci. 2023 Feb 9;24(4):3513. doi: 10.3390/ijms24043513. Int J Mol Sci. 2023. PMID: 36834922 Free PMC article. Review. - Deoxyhypusine synthase, an essential enzyme for hypusine biosynthesis, is required for proper exocrine pancreas development.
Padgett LR, Robertson MA, Anderson-Baucum EK, Connors CT, Wu W, Mirmira RG, Mastracci TL. Padgett LR, et al. FASEB J. 2021 May;35(5):e21473. doi: 10.1096/fj.201903177R. FASEB J. 2021. PMID: 33811703 Free PMC article.
References
- Chera S, et al. Silencing of the hydra serine protease inhibitor Kazal1 gene mimics the human SPINK1 pancreatic phenotype. J Cell Sci. 2006;119(Pt 5):846–57. - PubMed
- Mohrlen F, et al. Activation of pro-astacin. Immunological and model peptide studies on the processing of immature astacin, a zinc-endopeptidase from the crayfish Astacus astacus. Eur J Biochem. 2001;268(9):2540–6. - PubMed
- Nokhbatolfoghahai M, Downie JR. Amphibian hatching gland cells: pattern and distribution in anurans. Tissue Cell. 2007;39(4):225–40. - PubMed
- Inohaya K, et al. Temporal and spatial patterns of gene expression for the hatching enzyme in the teleost embryo, Oryzias latipes. Dev Biol. 1995;171(2):374–85. - PubMed
- Bonner-Weir S, et al. The pancreatic ductal epithelium serves as a potential pool of progenitor cells. Pediatr Diabetes. 2004;5(Suppl 2):16–22. - PubMed
Publication types
MeSH terms
Grants and funding
- T32 GM007814/GM/NIGMS NIH HHS/United States
- P30 DK079637/DK/NIDDK NIH HHS/United States
- DK61215/DK/NIDDK NIH HHS/United States
- R01 DK061215/DK/NIDDK NIH HHS/United States
- R01 DK056211/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources