Activation of the Hippo pathway by CTLA-4 regulates the expression of Blimp-1 in the CD8+ T cell - PubMed (original) (raw)
Activation of the Hippo pathway by CTLA-4 regulates the expression of Blimp-1 in the CD8+ T cell
James E D Thaventhiran et al. Proc Natl Acad Sci U S A. 2012.
Abstract
During the primary response, the commitment of the CD8(+) T cell to Blimp-1 expression and the terminal differentiation that Blimp-1 induces must be timed so as not to impair the process of clonal expansion. We determined whether the Hippo pathway, which links cell-cell contact to differentiation in other cell lineages, controls Blimp-1 expression. Activating the CD8(+) T cell with antigen and IL-2 causes expression of the core Hippo pathway components, including the pivotal transcriptional cofactor Yap. Contact between activated CD8(+) T cells induces Hippo pathway-mediated Yap degradation and Blimp-1 expression; a Hippo-resistant, stable form of Yap suppresses Blimp-1 expression. Cytotoxic T lymphocyte antigen 4 (CTLA-4) and CD80 comprise the receptor-ligand pair that mediates contact-dependent Hippo pathway activation. In vivo, CD8(+) T cells expressing Hippo resistant-Yap or lacking CTLA-4 have diminished expression of the senescence marker, KLRG1, during a viral infection. The CTLA-4/Hippo pathway/Blimp-1 system may couple terminal differentiation of CD8(+) T cell with the magnitude of clonal expansion.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
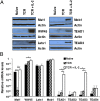
Expression of core components of the Hippo pathway by OT-I cells. (A) Immunoblot analysis of components of the Hippo pathway in whole-cell lysates prepared from naïve OT-I cells and OT-I cells stimulated for 24 h with 0.1 nM SIINFEKL peptide alone or with IL-2 (TCR + IL-2). (B) Bar graph showing the mRNA levels of these components in identically treated samples of OT-I cells relative to those levels for CD3ε (given an arbitrary level of one). *P < 0.05; ***P < 0.001. Data presented are the mean ± SEM (n = 3).
Fig. 2.
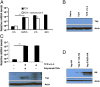
The regulation of Yap expression in OT-I cells. (A) Bar graph showing the mRNA levels, relative to the levels for CD3ε, for Yap in naive OT-I cells and OT-I cells stimulated for timed intervals with 0.1 nM SIINFEKL peptide in the absence or presence of actinomycin D. Data presented are the mean ± SEM. (B) Immunoblot analysis of Yap in whole-cell lysates prepared from naïve OT-I cells and OT-I cells stimulated for 24 h with 0.1 nM SIINFEKL peptide alone (TCR) or together with IL-2 (TCR + IL-2). (C) Levels of Yap protein (Lower) and Yap mRNA levels (Upper) in whole-cell lysates prepared from naïve Thy1.2+ OT-I cells and Thy1.2+ OT-I cells that had been stimulated with SIINFEKL peptide and IL-2 for 24 h alone or in the presence of 10-fold excess of nonactivated Thy1.1+ C57BL/6 CD8+ T cells. The Thy1.2+ OT-I cells were recovered by MACS purification, and Yap protein and mRNA were assessed. Data presented are the mean ± SEM. (D) Immunoblot analysis using antihemagglutin (anti-HA) of whole-cell lysates prepared from retrovirally transduced OT-I cells that expressed ectopic WT Yap tagged with HA (Yap-HA) and Yap in which serine 112 had been mutated to alanine (Yap S112A-HA) or serine-382 had been mutated to alanine (Yap S382A-HA). Lysates were cultured for 48 h with IL-2. ***P < 0.001.
Fig. 3.
The role of CD80/86 in triggering the Hippo pathway during contact between activated CD8+ T cells. (A) Bar graph showing the mRNA levels of CD80 and CD86 relative to the levels of CD3ε in naïve OT-I cells and OT-I cells stimulated for 24 h with 0.1 nM SIINFEKL peptide alone (TCR) or together with IL-2 (TCR + IL-2). Data presented are the mean ± SEM. (B) Naïve and TCR + IL-2–stimulated OT-I cells were also assessed by FACS for membrane expression of CD80 and CD86 (shaded histogram, isotype control; black line, specific antibody). (C) Immunoblot analysis of Yap in cell lysates prepared from naïve OT-I cells or OT-I cells that had been stimulated for 24 h with SIINFEKL peptide in the absence or presence IL-2 and the presence of isotype control antibodies or blocking antibodies to CD80 and CD86. (D) Analysis by confocal microscopy of Yap expression by naïve OT-I cells or OT-I cells that had been stimulated for 24 h with SIINFEKL peptide and IL-2 in the presence of isotype control antibodies or blocking antibodies to CD80 and CD86 (green, anti-Yap; blue, DAPI). (Scale bars: 50 μm.) ***P < 0.001.
Fig. 4.
Plasma membrane expression of CTLA-4 requires contact between activated CD8+ T cells. (A) Bar graph showing the mRNA levels of CTLA-4 relative to the levels for CD3ε in naïve OT-I cells and OT-I cells stimulated for 24 h with 0.1 nM SIINFEKL peptide alone (TCR) or together with IL-2 (TCR + IL-2). Data presented are the mean ± SEM. (B) Immunoblots showing CTLA-4 and actin protein in lysates of naïve OT-I cells and OT-I cells stimulated for 24 h with 0.1 nM SIINFEKL peptide alone (TCR) or together with IL-2 (TCR + IL-2). (C) FACS analysis of CTLA-4 expression by TCR- and IL-2–stimulated Thy1.2+ OT-I cells that had been cocultured for 30 h alone or with increasing numbers of resting polyclonal Thy1.1+ CD8+ T cells. The numbers represent the proportion of intact and detergent-permeabilized Thy1.2+ cells, respectively, showing specific staining with anti–CTLA-4. (D) FACS analysis of CTLA-4 expression by TCR and IL-2–stimulated Thy1.2+ OT-I cells that had been cultured in the top compartment of a transwell chamber in which the bottom compartment contained activated Thy1.2+ OT-I cells cocultured with 100-fold excess polyclonal CD8+ T cells. The numbers represent the proportion of intact, nonpermeabilized Thy1.2+ cells staining with anti–CTLA-4. *P < 0.05; ***P < 0.001.
Fig. 5.
Activation of the Hippo pathway by CTLA-4 in vitro and in vivo. (A) Immunoblot analysis of lysates from activated OT-I cells cultured in the presence of antibodies to CD80 and CD86 and incubated for 1 h with beads bearing isotype control antibody or antibody to CTLA-4. Blots were developed with antibodies specific for phosphothreonine-183 of Mst1 and phosphoserine-909 of Lats1. Staining of actin is shown as a loading control. (B) Immunoblot analysis with antiphosphoserine-112 of Yap of lysates from activated OT-I cells expressing Yap S382A-HA that had been cultured in the presence of blocking antibodies to CD80 and CD86 and incubated for 1 h with beads bearing isotype control antibody or antibody to CTLA-4. HA staining is shown as a loading control. (C) Immunoblot analysis of WT Yap in cell lysates from OT-I cells activated in the presence of antibodies to CD80 and CD86 and incubated for 3 h with beads bearing various ratios of isotype control antibody and antibody to CTLA-4. Staining of actin is shown as a loading control. (D) Analysis by confocal microscopy of anti-Yap–stained OT-I cells that had been activated in the presence of blocking antibodies to CD80 and CD86 and incubated for 3 h with beads bearing isotype control antibody or antibody to CTLA-4 (green, anti-Yap; blue, DAPI). (Scale bars: 50 μm.) (E) Immunoblot analysis of Yap protein in lysates from CTLA-4−/− OT-I cells and WT OT-I cells that had been activated with SIINFEKL peptide and IL-2 for 24 h. (F) Analysis by confocal microscopy of Yap protein in CD8+ and CD4+ cells from the lymph nodes of CTLA-4−/− and CTLA-4−/+ 16- to 20-d-old mice. (Scale bars: 10 μm.)
Fig. 6.
Preventing Hippo pathway activation in CD8+ T cells affects Blimp-1 expression and differentiation. (A) OT-I CD8+ T cells were transduced with the Yap 5SA-expressing retrovirus or the pMig vector control and analyzed for Eomes protein by staining of permeabilized cells with anti-Eomes relative to naïve, unstimulated OT-I cells 16 h after restimulation with IL-2 and SIINFEKL peptide. Numbers represent the Eomes-specific mean fluorescence intensity ± SEM. (B) Prdm1gfp/+ CD8+ T cells that had been transduced with control pMig (black line) or the pMig vector-expressing Yap 5SA (red line) and WT CD8+ T cells transduced with pMig (shaded) were restimulated for 48 h with anti-CD3ε and IL-2 and assessed for GFP fluorescence. Numbers are the GFP-specific mean fluorescence intensities ± SEM. (C and D) OT-I cells transduced with a retrovirus expressing the Lats-resistant Yap 5SA or pMig control vector (C) and CTLA-4+/+ or CTLA-4−/− OT-I cells (D) were analyzed for Blimp-1 mRNA 48 h after restimulation with IL-2 and SIINFEKL peptide. The levels of Blimp-1 mRNA relative to the levels of naïve unstimulated OT-I cells are shown. Data presented are the mean ± SEM. (E and F) Thy1.2+ OT-I cells that had been transduced with the Yap 5SA-expressing retrovirus or control pMig (E), and Thy1.2+ CTLA-4−/− OT-I cells or CTLA-4+/+ OT-I cells (F) were adoptively transferred to Thy1.1+ C57BL/6 mice, and 2 d later, the mice were infected with γ-MHV-68/OVA. On days 8 and 11 postinfection, peripheral blood Thy1.2+ OT-I cells were assessed for expression of KLRG1. Numbers represent the percentage KLRG1+ cells (means ± SEM). *P < 0.05.
Fig. P1.
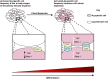
Quorum sensing model of CD8+ T-cell differentiation. Clonal expansion increases the frequency of activated antigen-specific cells and the likelihood of cell–cell contact leading to the surface expression and ligation of CTLA-4 to CD80, which triggers differentiation.
Similar articles
- Manipulating Memory CD8 T Cell Numbers by Timed Enhancement of IL-2 Signals.
Kim MT, Kurup SP, Starbeck-Miller GR, Harty JT. Kim MT, et al. J Immunol. 2016 Sep 1;197(5):1754-61. doi: 10.4049/jimmunol.1600641. Epub 2016 Jul 20. J Immunol. 2016. PMID: 27439516 Free PMC article. - Cytokine-dependent Blimp-1 expression in activated T cells inhibits IL-2 production.
Gong D, Malek TR. Gong D, et al. J Immunol. 2007 Jan 1;178(1):242-52. doi: 10.4049/jimmunol.178.1.242. J Immunol. 2007. PMID: 17182561 - Quorum Regulation via Nested Antagonistic Feedback Circuits Mediated by the Receptors CD28 and CTLA-4 Confers Robustness to T Cell Population Dynamics.
Zenke S, Palm MM, Braun J, Gavrilov A, Meiser P, Böttcher JP, Beyersdorf N, Ehl S, Gerard A, Lämmermann T, Schumacher TN, Beltman JB, Rohr JC. Zenke S, et al. Immunity. 2020 Feb 18;52(2):313-327.e7. doi: 10.1016/j.immuni.2020.01.018. Epub 2020 Feb 11. Immunity. 2020. PMID: 32049052 - CTLA-4 and T cell activation.
Oosterwegel MA, Greenwald RJ, Mandelbrot DA, Lorsbach RB, Sharpe AH. Oosterwegel MA, et al. Curr Opin Immunol. 1999 Jun;11(3):294-300. doi: 10.1016/s0952-7915(99)80047-8. Curr Opin Immunol. 1999. PMID: 10375557 Review. - Molecular and Cellular Functions of CTLA-4.
Van Coillie S, Wiernicki B, Xu J. Van Coillie S, et al. Adv Exp Med Biol. 2020;1248:7-32. doi: 10.1007/978-981-15-3266-5_2. Adv Exp Med Biol. 2020. PMID: 32185705 Review.
Cited by
- Influence of the Hippo-YAP signalling pathway on tumor associated macrophages (TAMs) and its implications on cancer immunosuppressive microenvironment.
Yang W, Yang S, Zhang F, Cheng F, Wang X, Rao J. Yang W, et al. Ann Transl Med. 2020 Mar;8(6):399. doi: 10.21037/atm.2020.02.11. Ann Transl Med. 2020. PMID: 32355843 Free PMC article. Review. - The Ikaros transcription factor regulates responsiveness to IL-12 and expression of IL-2 receptor alpha in mature, activated CD8 T cells.
Clambey ET, Collins B, Young MH, Eberlein J, David A, Kappler JW, Marrack P. Clambey ET, et al. PLoS One. 2013;8(2):e57435. doi: 10.1371/journal.pone.0057435. Epub 2013 Feb 26. PLoS One. 2013. PMID: 23483882 Free PMC article. - Mechanosensing through YAP controls T cell activation and metabolism.
Meng KP, Majedi FS, Thauland TJ, Butte MJ. Meng KP, et al. J Exp Med. 2020 Aug 3;217(8):e20200053. doi: 10.1084/jem.20200053. J Exp Med. 2020. PMID: 32484502 Free PMC article. - Quorum sensing in the immune system.
Antonioli L, Blandizzi C, Pacher P, Guilliams M, Haskó G. Antonioli L, et al. Nat Rev Immunol. 2018 Sep;18(9):537-538. doi: 10.1038/s41577-018-0040-4. Nat Rev Immunol. 2018. PMID: 30006523 Free PMC article. - Biological Significance of YAP/TAZ in Pancreatic Ductal Adenocarcinoma.
Hayashi H, Uemura N, Zhao L, Matsumura K, Sato H, Shiraishi Y, Baba H. Hayashi H, et al. Front Oncol. 2021 Jul 29;11:700315. doi: 10.3389/fonc.2021.700315. eCollection 2021. Front Oncol. 2021. PMID: 34395269 Free PMC article. Review.
References
- Kallies A, Xin A, Belz GT, Nutt SL. Blimp-1 transcription factor is required for the differentiation of effector CD8(+) T cells and memory responses. Immunity. 2009;31:283–295. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials