Landscape of somatic retrotransposition in human cancers - PubMed (original) (raw)
. 2012 Aug 24;337(6097):967-71.
doi: 10.1126/science.1222077. Epub 2012 Jun 28.
Rebecca Iskow, Lixing Yang, Omer Gokcumen, Psalm Haseley, Lovelace J Luquette 3rd, Jens G Lohr, Christopher C Harris, Li Ding, Richard K Wilson, David A Wheeler, Richard A Gibbs, Raju Kucherlapati, Charles Lee, Peter V Kharchenko, Peter J Park; Cancer Genome Atlas Research Network
Affiliations
- PMID: 22745252
- PMCID: PMC3656569
- DOI: 10.1126/science.1222077
Landscape of somatic retrotransposition in human cancers
Eunjung Lee et al. Science. 2012.
Abstract
Transposable elements (TEs) are abundant in the human genome, and some are capable of generating new insertions through RNA intermediates. In cancer, the disruption of cellular mechanisms that normally suppress TE activity may facilitate mutagenic retrotranspositions. We performed single-nucleotide resolution analysis of TE insertions in 43 high-coverage whole-genome sequencing data sets from five cancer types. We identified 194 high-confidence somatic TE insertions, as well as thousands of polymorphic TE insertions in matched normal genomes. Somatic insertions were present in epithelial tumors but not in blood or brain cancers. Somatic L1 insertions tend to occur in genes that are commonly mutated in cancer, disrupt the expression of the target genes, and are biased toward regions of cancer-specific DNA hypomethylation, highlighting their potential impact in tumorigenesis.
Figures
Fig. 1
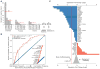
(A) To detect somatic insertions of TEs, paired-end sequencing data from tumor and matched normal samples are aligned to both the reference genome and a custom repeat assembly of canonical and divergent TE sequences. Two types of supporting reads are identified: (i) repeat-anchored mate (RAM) reads, in which one of the paired-end reads is mapped to a unique location in the genome, whereas the other is associated with a TE (reads 1 to 4), and (ii) clipped reads, which span the TE insertion breakpoints and show partial alignment to the reference or the repeat assembly (reads 5 to 8). The distances between the clipping positions and the clipped sequences are used to infer the insertion mechanism. For instance, duplicated sequences at the insertion site (TSD) and the poly-A tail of the inserted TE are characteristics of an endonuclease-mediated target-primed retrotransposition. (B) Example: a validated somatic L1 insertion in the 3′ UTR of GPATCH2 in colorectal cancer (CR3518). The top chart displays two clusters of RAM reads (green) whose mate pairs (not shown) are associated with L1 repeat sequences. Clipped (partially aligned) reads spanning the insertion breakpoint are shown underneath, with each nucleotide in a different color (nucleotides matching the reference are not shown). The consecutive red bases to the right of the insertion come from the poly-T tail of the inserted L1 in the negative orientation. The separation of clipped read positions between the strands reveals a 19-bp TSD (bottom). No RAMs or clipped reads are observed in the matched normal (blood) sample.
Fig. 2
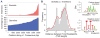
(A) Frequency of high-confidence somatic L1 insertions varies across 5 colorectal, 7 prostate, 8 ovarian, 7 multiple myeloma, and 16 glioblastoma tumors. Three epithelial cancers (colorectal, prostate, and ovarian) show frequent somatic L1 insertions, whereas no insertions are observed in the blood and brain cancers. One colorectal tumor (CR3518) contains 102 L1 insertions, increasing the average somatic event frequency for colorectal tumors from 9 to 28 when this sample is included. (B) The genes affected by somatic TE insertions are significantly enriched for genes with high mutation rates as estimated from the exome sequencing data of 228 additional colorectal tumors (P < 1 × 10−15). The mutation frequency of each gene was adjusted for its total exon size. (Inset) The top 15 genes with nonsilent mutations. (C) The transcript levels of 45 genes with somatic TE insertions in colorectal tumors were compared with those from 28 normal colorectal tissues, and the expression fold changes are shown. Overall, the genes with a TE insertion were significantly down-regulated in tumors (P = 6.3 × 10−4, background distribution based on randomly sampled gene sets). KCNIP1 appears twice because of two somatic insertions in two different samples. The dashed line marks 50% reduction in expression.
Fig. 3
(A) Most of the identified insertions do not contain a full L1 sequence (6 kbp) but are truncated at the 5′ end. The parts of the L1 sequence found within the identified somatic and germline insertions are illustrated as a coverage plot. (B) A positive distance between the clipping positions of clipped reads with negative-and positive-strand mapping (Fig. 1B) corresponds to the length of the duplicated sequence at a TSD, whereas a negative or zero distance corresponds to a microdeletion or lack of duplication at the insertion site. The major TSD peak at ~15 bp is characteristic of an endonuclease-dependent L1 retrotransposition. Sequence analysis around the insertion breakpoints revealed the 5′-TTTT/A-3′ (where the slash indicates the insertion breakpoint) motif, consistent with a canonical sequence for L1-endonuclease target sites (31). The insertions belonging to the minor TSD peak (0 to 2 bp) did not show a significant sequence motif.
Fig. 4
(A) Somatic L1 insertions are biased toward hypomethylated regions in cancer cells. Colon cancer regions were assessed in independent samples. (B) A model of TE insertion preferences and subsequent selection process that bias genomic distribution of TE insertions is illustrated. Somatic insertions are strongly biased toward cancer-specific DNA hypomethylation regions (red box) and encounter selection that depletes them from transcriptionally active genes unless such insertions promote tumorigenesis. By contrast, germline insertions are biased toward germline-specific DNA hypomethylation domains (blue box) and are depleted from all genes.
Similar articles
- Extensive somatic L1 retrotransposition in colorectal tumors.
Solyom S, Ewing AD, Rahrmann EP, Doucet T, Nelson HH, Burns MB, Harris RS, Sigmon DF, Casella A, Erlanger B, Wheelan S, Upton KR, Shukla R, Faulkner GJ, Largaespada DA, Kazazian HH Jr. Solyom S, et al. Genome Res. 2012 Dec;22(12):2328-38. doi: 10.1101/gr.145235.112. Epub 2012 Sep 11. Genome Res. 2012. PMID: 22968929 Free PMC article. - Immune signatures correlate with L1 retrotransposition in gastrointestinal cancers.
Jung H, Choi JK, Lee EA. Jung H, et al. Genome Res. 2018 Aug;28(8):1136-1146. doi: 10.1101/gr.231837.117. Epub 2018 Jul 3. Genome Res. 2018. PMID: 29970450 Free PMC article. - Frequent L1 retrotranspositions originating from TTC28 in colorectal cancer.
Pitkänen E, Cajuso T, Katainen R, Kaasinen E, Välimäki N, Palin K, Taipale J, Aaltonen LA, Kilpivaara O. Pitkänen E, et al. Oncotarget. 2014 Feb 15;5(3):853-9. doi: 10.18632/oncotarget.1781. Oncotarget. 2014. PMID: 24553397 Free PMC article. - All y'all need to know 'bout retroelements in cancer.
Belancio VP, Roy-Engel AM, Deininger PL. Belancio VP, et al. Semin Cancer Biol. 2010 Aug;20(4):200-10. doi: 10.1016/j.semcancer.2010.06.001. Epub 2010 Jun 25. Semin Cancer Biol. 2010. PMID: 20600922 Free PMC article. Review. - The Role of Somatic L1 Retrotransposition in Human Cancers.
Scott EC, Devine SE. Scott EC, et al. Viruses. 2017 May 31;9(6):131. doi: 10.3390/v9060131. Viruses. 2017. PMID: 28561751 Free PMC article. Review.
Cited by
- The promise and failures of epigenetic therapies for cancer treatment.
Bojang P Jr, Ramos KS. Bojang P Jr, et al. Cancer Treat Rev. 2014 Feb;40(1):153-69. doi: 10.1016/j.ctrv.2013.05.009. Epub 2013 Jul 5. Cancer Treat Rev. 2014. PMID: 23831234 Free PMC article. Review. - Robust expression of LINE-1 retrotransposon encoded proteins in oral squamous cell carcinoma.
Mukherjee K, Sur D, Singh A, Rai S, Das N, Sekar R, Narindi S, Dhingra VK, Jat B, Balraam KVV, Agarwal SP, Mandal PK. Mukherjee K, et al. BMC Cancer. 2021 May 27;21(1):628. doi: 10.1186/s12885-021-08174-z. BMC Cancer. 2021. PMID: 34044801 Free PMC article. - RNA m6A modification orchestrates a LINE-1-host interaction that facilitates retrotransposition and contributes to long gene vulnerability.
Xiong F, Wang R, Lee JH, Li S, Chen SF, Liao Z, Hasani LA, Nguyen PT, Zhu X, Krakowiak J, Lee DF, Han L, Tsai KL, Liu Y, Li W. Xiong F, et al. Cell Res. 2021 Aug;31(8):861-885. doi: 10.1038/s41422-021-00515-8. Epub 2021 Jun 9. Cell Res. 2021. PMID: 34108665 Free PMC article. - Long interspersed element-1 (LINE-1): passenger or driver in human neoplasms?
Rodić N, Burns KH. Rodić N, et al. PLoS Genet. 2013 Mar;9(3):e1003402. doi: 10.1371/journal.pgen.1003402. Epub 2013 Mar 28. PLoS Genet. 2013. PMID: 23555307 Free PMC article. Review. - Human immunodeficiency virus, herpes virus infections, and pulmonary vascular disease.
Flores SC, Almodovar S. Flores SC, et al. Pulm Circ. 2013 Jan;3(1):165-70. doi: 10.4103/2045-8932.109955. Pulm Circ. 2013. PMID: 23662195 Free PMC article.
References
- Slotkin RK, Martienssen R. Nat Rev Genet. 2007;8:272. - PubMed
- Yang N, Kazazian HH., Jr Nat Struct Mol Biol. 2006;13:763. - PubMed
- Prak ETL, Kazazian HH., Jr Nat Rev Genet. 2000;1:134. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- K25 AG037596/AG/NIA NIH HHS/United States
- R01GM082798/GM/NIGMS NIH HHS/United States
- R01 GM082798/GM/NIGMS NIH HHS/United States
- U24CA144025/CA/NCI NIH HHS/United States
- U54 HG003273/HG/NHGRI NIH HHS/United States
- RC1HG005482/HG/NHGRI NIH HHS/United States
- U01HG005725/HG/NHGRI NIH HHS/United States
- K25AG037596/AG/NIA NIH HHS/United States
- U01 HG005209/HG/NHGRI NIH HHS/United States
- F32AG039979/AG/NIA NIH HHS/United States
- F32 AG039979/AG/NIA NIH HHS/United States
- T32 CA009172/CA/NCI NIH HHS/United States
- U24 CA144025/CA/NCI NIH HHS/United States
- RC1 HG005482/HG/NHGRI NIH HHS/United States
- U01HG005209/HG/NHGRI NIH HHS/United States
- U01 HG005725/HG/NHGRI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical