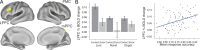Global connectivity of prefrontal cortex predicts cognitive control and intelligence - PubMed (original) (raw)
Global connectivity of prefrontal cortex predicts cognitive control and intelligence
Michael W Cole et al. J Neurosci. 2012.
Abstract
Control of thought and behavior is fundamental to human intelligence. Evidence suggests a frontoparietal brain network implements such cognitive control across diverse contexts. We identify a mechanism--global connectivity--by which components of this network might coordinate control of other networks. A lateral prefrontal cortex (LPFC) region's activity was found to predict performance in a high control demand working memory task and also to exhibit high global connectivity. Critically, global connectivity in this LPFC region, involving connections both within and outside the frontoparietal network, showed a highly selective relationship with individual differences in fluid intelligence. These findings suggest LPFC is a global hub with a brainwide influence that facilitates the ability to implement control processes central to human intelligence.
Figures
Figure 1.
Cognitive control regions, as defined by successful cognitive control. A, ROIs were defined based on brain activity during successful N-back task performance. The following highly selective criteria were used: preferential activation for trials requiring flexible control (lures), correct > incorrect trials, positive correlation with accuracy across participants. All 3 of these regions were hubs (in top 10% connectivity in the brain). B, Illustration of effects defining the LPFC ROI. LPFC was more active across correct relative to error trials and more active for lure than other trial types. LPFC activity (correct trials across all trial types) was also correlated with overall response accuracy across individuals (r = 0.39, p = 0.0002).
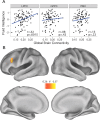
Figure 2.
GBC correlations with gF. A, Positive GBC was calculated for the 3 regions identified in the task-based analysis, and correlations were assessed with gF. Only LPFC showed a significant correlation. GBC is a graph theoretical measure of resting state fcMRI, calculated here for each seed ROI, by computing the average connectivity strength between the region and every other voxel of the brain. Note that all 3 regions were in the top 10% in terms of GBC (but only LPFC GBC was significantly correlated with gF). B, Correlations were assessed between each brain voxel's positive GBC and gF scores. Of the entire brain, only left LPFC was statistically significant (p < 0.05, corrected for multiple comparisons). The LPFC region strongly overlapped with the LPFC region identified in the task-based analysis.
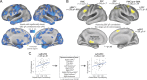
Figure 3.
Examining the specificity of the left LPFC GBC–gF correlation. A, The LPFC GBC–gF correlation was compared with the GBC–gF correlation of every brain voxel. Significant voxel clusters are shown (p < 0.05, corrected for multiple comparisons). This supports the specificity of the LPFC GBC–gF effect and demonstrates that the contribution of global connectivity to intelligence is not uniform throughout the brain. Potential control regions (based on previous studies) are highlighted. B, The GBC–gF correlation was calculated for a larger set of regions from Burgess et al. (2011) that appeared likely to show GBC–gF correlations. Using the same dataset as here, Burgess et al. (2011) identified 10 regions (the largest 8 are depicted here) with N-back lure fMRI activity correlating with individual differences in cognitive control capacity (e.g., gF). Despite these regions' activities correlating with gF, only left LPFC (substantially overlapping with the previously defined LPFC region) showed a significant GBC–gF correlation. C, The left LPFC GBC–gF correlation was computed before and after removing the variance from all Burgess et al. (2011) regions (excluding left LPFC). The GBC–gF correlation for the left LPFC region (as defined in Fig. 1) was largely unaffected by removing the variance from the Burgess et al. (2011) regions. This demonstrates that the left LPFC GBC–gF correlation was statistically independent of a large set of cognitive control regions. Note that 9 of the 10 regions had voxels in the top 10% GBC, demonstrating that the GBC–gF correlation is not guaranteed simply due to a control region having both gF-correlating activity and high GBC.
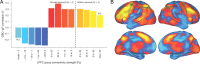
Figure 4.
Identifying the origin of the LPFC GBC–gF correlation. A, We systematically restricted the connectivity contributing to the calculation of the GBC–gF correlation to determine the contributing connectivity strength ranges. LPFC's group average connectivity map was split into Fz 0.05 “strength bands” and restricted GBC–gF correlations calculated using those voxels. All strength ranges were significant, except the highest and lowest, likely due to the low number of voxels included in these 2 strength ranges. This likely indicates that the GBC–gF effect is global in origin, such that the more voxels that are included the better the connectivity–gF correlation. This is in contrast to the effect originating from high-strength connectivity, in which restricting to higher strength connections would improve the connectivity–gF correlation. B, The LPFC seed connectivity group map is shown, colored by connectivity strength ranges (corresponding to colors in A).
Figure 5.
Testing for global or system-specific origins of the LPFC GBC–gF effect. A, We separated LPFC connectivity into 3 systems. The cognitive control network was identified using a meta-analysis of cognitive control studies, sensory-motor regions were identified based on probabilistic cytoarchitecture, and the default mode network was identified using a meta-analysis of studies investigating that network. B, LPFC group average connectivity generally had high positive strength for cognitive control regions, low positive and negative strength (combined using absolute values) for sensory-motor regions, and high negative strength for default mode regions. We used the conjunctions between the masks in A along with these group strength ranges to isolate the 3 systems. Individual differences in mean LPFC connectivity with each of the 3 systems correlated with gF, suggesting a truly global origin of the LPFC GBC–gF effect.
Similar articles
- Lateral Prefrontal Cortex Contributes to Fluid Intelligence Through Multinetwork Connectivity.
Cole MW, Ito T, Braver TS. Cole MW, et al. Brain Connect. 2015 Oct;5(8):497-504. doi: 10.1089/brain.2015.0357. Epub 2015 Sep 23. Brain Connect. 2015. PMID: 26165732 Free PMC article. - Transfer of learning relates to intrinsic connectivity between hippocampus, ventromedial prefrontal cortex, and large-scale networks.
Gerraty RT, Davidow JY, Wimmer GE, Kahn I, Shohamy D. Gerraty RT, et al. J Neurosci. 2014 Aug 20;34(34):11297-303. doi: 10.1523/JNEUROSCI.0185-14.2014. J Neurosci. 2014. PMID: 25143610 Free PMC article. - Temporally anticorrelated brain networks during working memory performance reveal aberrant prefrontal and hippocampal connectivity in patients with schizophrenia.
Wolf RC, Vasic N, Sambataro F, Höse A, Frasch K, Schmid M, Walter H. Wolf RC, et al. Prog Neuropsychopharmacol Biol Psychiatry. 2009 Nov 13;33(8):1464-73. doi: 10.1016/j.pnpbp.2009.07.032. Epub 2009 Aug 8. Prog Neuropsychopharmacol Biol Psychiatry. 2009. PMID: 19666074 - Brain-wide resting-state connectivity regulation by the hippocampus and medial prefrontal cortex is associated with fluid intelligence.
Li R, Zhang J, Wu X, Wen X, Han B. Li R, et al. Brain Struct Funct. 2020 Jun;225(5):1587-1600. doi: 10.1007/s00429-020-02077-8. Epub 2020 Apr 24. Brain Struct Funct. 2020. PMID: 32333100 - Connectivity patterns in cognitive control networks predict naturalistic multitasking ability.
Wen T, Liu DC, Hsieh S. Wen T, et al. Neuropsychologia. 2018 Jun;114:195-202. doi: 10.1016/j.neuropsychologia.2018.05.002. Epub 2018 May 2. Neuropsychologia. 2018. PMID: 29729277 Free PMC article.
Cited by
- Distinct functional and structural connections predict crystallised and fluid cognition in healthy adults.
Dhamala E, Jamison KW, Jaywant A, Dennis S, Kuceyeski A. Dhamala E, et al. Hum Brain Mapp. 2021 Jul;42(10):3102-3118. doi: 10.1002/hbm.25420. Epub 2021 Apr 8. Hum Brain Mapp. 2021. PMID: 33830577 Free PMC article. - Distributed functional connectivity predicts neuropsychological test performance among older adults.
Kwak S, Kim H, Kim H, Youm Y, Chey J. Kwak S, et al. Hum Brain Mapp. 2021 Jul;42(10):3305-3325. doi: 10.1002/hbm.25436. Epub 2021 May 7. Hum Brain Mapp. 2021. PMID: 33960591 Free PMC article. - Emulative, coherent, and causal dynamics between large-scale brain networks are neurobiomarkers of Accelerated Cognitive Ageing in epilepsy.
Bernas A, Breuer LEM, Aldenkamp AP, Zinger S. Bernas A, et al. PLoS One. 2021 Apr 16;16(4):e0250222. doi: 10.1371/journal.pone.0250222. eCollection 2021. PLoS One. 2021. PMID: 33861794 Free PMC article. - Task-induced activation transmitted by structural connectivity is associated with behavioral performance.
Yan T, Liu T, Ai J, Shi Z, Zhang J, Pei G, Wu J. Yan T, et al. Brain Struct Funct. 2021 Jun;226(5):1437-1452. doi: 10.1007/s00429-021-02249-0. Epub 2021 Mar 20. Brain Struct Funct. 2021. PMID: 33743076 - Frontoparietal network topology as a neural marker of musical perceptual abilities.
Lumaca M, Keller PE, Baggio G, Pando-Naude V, Bajada CJ, Martinez MA, Hansen JH, Ravignani A, Joe N, Vuust P, Vulić K, Sandberg K. Lumaca M, et al. Nat Commun. 2024 Sep 17;15(1):8160. doi: 10.1038/s41467-024-52479-z. Nat Commun. 2024. PMID: 39289390 Free PMC article.
References
- Åström KJ, Murray RM. Feedback systems: an introduction for scientists and engineers. Princeton, NJ: Princeton UP; 2008.
- Braver TS, Cohen JD, Nystrom LE, Jonides J, Smith EE, Noll DC. A parametric study of prefrontal cortex involvement in human working memory. Neuroimage. 1997;5:49–62. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- MH66078-06A1W1/MH/NIMH NIH HHS/United States
- NR012081/NR/NINR NIH HHS/United States
- R37 MH066078/MH/NIMH NIH HHS/United States
- DP5 OD012109/OD/NIH HHS/United States
- F32 NR012081/NR/NINR NIH HHS/United States
- MH66088/MH/NIMH NIH HHS/United States
- R56 MH066078/MH/NIMH NIH HHS/United States
- R01 MH066088/MH/NIMH NIH HHS/United States
- MH66078/MH/NIMH NIH HHS/United States
- K99 MH096801/MH/NIMH NIH HHS/United States
- R01 MH066078/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources