External push and internal pull forces recruit curvature-sensing N-BAR domain proteins to the plasma membrane - PubMed (original) (raw)
External push and internal pull forces recruit curvature-sensing N-BAR domain proteins to the plasma membrane
Milos Galic et al. Nat Cell Biol. 2012 Aug.
Abstract
Many of the more than 20 mammalian proteins with N-BAR domains control cell architecture and endocytosis by associating with curved sections of the plasma membrane. It is not well understood whether N-BAR proteins are recruited directly by processes that mechanically curve the plasma membrane or indirectly by plasma-membrane-associated adaptor proteins that recruit proteins with N-BAR domains that then induce membrane curvature. Here, we show that externally induced inward deformation of the plasma membrane by cone-shaped nanostructures (nanocones) and internally induced inward deformation by contracting actin cables both trigger recruitment of isolated N-BAR domains to the curved plasma membrane. Markedly, live-cell imaging in adherent cells showed selective recruitment of full-length N-BAR proteins and isolated N-BAR domains to plasma membrane sub-regions above nanocone stripes. Electron microscopy confirmed that N-BAR domains are recruited to local membrane sites curved by nanocones. We further showed that N-BAR domains are periodically recruited to curved plasma membrane sites during local lamellipodia retraction in the front of migrating cells. Recruitment required myosin-II-generated force applied to plasma-membrane-connected actin cables. Together, our results show that N-BAR domains can be directly recruited to the plasma membrane by external push or internal pull forces that locally curve the plasma membrane.
Figures
Figure 1. Nanocones induce inward curved PM deformations at the basal PM of adherent cells
(a) Scanning electron micrographs of Nanocones on glass substrate shown from the top (top, left) and the side (top, right). SEM of 3T3 cells (red) grown on 200nm Nanocones (bottom, left). Transparency of Nanocones decreases with increasing cone height (bottom, right). For each height, transparency was measured at 8 different regions of the sample. Error bars represent s.e.m. of the mean value. (b) 3T3 cells cultured on 20μm wide stripes of 600nm Nanocones (top) and 200nm Nanocones (bottom). The sawtooth symbols at the bottom of the panels and the white lines depict the location of the Nanocone stripes. After 48h cell density on 600nm cones (brown) is significantly lower compared to adjacent glass surface while no changes are visible for 200nm Nanocones (yellow). Analysis of cell density for Nanocones of 600nm (n=1484 cells, top) and 200mn (n=780 cells, bottom) height are shown to the right. (c) Scanning electron micrographs of the inner side of the PM of cells grown on 200nm Nanocones. Cells were sonicated to remove all but the PM of cells grown on Nanocones (red). Note the Nanocone-induced deformation of the PM (d) Transmitted electron micrographs of 3T3 cells grown on 200nm Nanocones. Cells are colored in red. Note that Nanocones do not penetrate the PM. Scale bars; (a), top panels 200nm, bottom panel 1μm; (b), 20 μm; (c), top left panel 10 μm, all other 200nm; (d), top panel 5 μm, bottom panels 200nm.
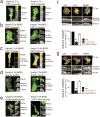
Figure 2. Nanocone-induced membrane deformation triggers N-BAR domain recruitment to the PM
(a) Nadrin2 forms puncta over Nanocones. 3T3 cells were grown on 3μm wide stripes of Nanocones and transfected with Nadrin2 (red) together with membrane marker CAAX (green). Nadrin2 puncta formation was selectively observed over Nanocone stripes. Magnification of a selected region (white box) is shown next to it. (b, c) The N-BAR domain is sufficient for puncta formation. Quantitative analysis of the increase in puncta density induced by Nanocones for full length (FL, light red, n=14 cells) and the isolated N-BAR domain (N-BAR, dark red, n=12 cells) of Nadrin2 (b) and full length (FL, light red, n=10 cells) and the isolated N-BAR domain (N-BAR, dark red, n=12 cells) of Amphiphysin1 (c), respectively. Error bars represent s.e.m. of the mean value (d) Transmitted electron micrograph show that the N-BAR domain of Nadrin2 accumulates on membrane deformations induced by Nanocones. 3T3 cells were transfected with fluorescently tagged N-BAR domain of Nadrin2 fixed and incubated with primary antibody directed against the fluorescent tag. Note that gold-conjugated secondary antibody accumulate over Nanocone-induced inward membrane deformations. Immunogold density was measured over Nanocones (n = 25) where deformation of the PM was observed and compared to adjacent regions within the same image. Error bars represent s.e.m. of the mean value. (e) Nanocone-induced N-BAR domain puncta of Nadrin2 are stationary and long-lived. 3T3 cell plated on Nanocones was transfected with fluorescently tagged N-BAR domain of Nadrin2 and imaged every 500ms for 60 seconds. Kymograph of two representative puncta (orange) is shown below. To the right, a graph depicting traces of individual N-BAR domain puncta (red), cytosol (green) and background (black) are shown. (f) FRAP analysis indicates a stable, long-lasting and a dynamic fraction of comparable sizes over individual Nanocones. Magnified area showing a time-series following a single Nanocone-induced N-BAR domain accumulation through a FRAP cycle (top) and analysis of multiple experiments (bottom) are shown. Scale bars (a-c), 3 μm; (d), 200nm; (e), 10μm; (f), 1 μm; ** P < 0.01.
Figure 3. N-BAR domains are dynamically recruited to local membrane sites at the leading edge of migrating 3T3 cells
(a) The proteins Nadrin2 and Amphiphysin1 are enriched in the leading edge of migrating 3T3 cells. (b) Enrichment in the leading edge is dependent on the N-BAR domain. Cells expressing a cytosolic marker (CFP, green) and the isolated N-BAR domain of Nadrin2 (left, red) and Amphiphysin1 (right, red). Note the polarized localization of the isolated N-BAR domain to the leading edge. (c) Enrichment in the leading edge is dependent on the amphipatic helix. Cells expressing a cytosolic marker (CFP, green) and the isolated N-BAR domain of Nadrin2 (left, red) and Amphiphysin1 (right, red) that is lacking the amino-terminal amphipatic helix show no polarized localization to the leading edge. (d) N-BAR domain patches show significant overlap with marker for filamentous actin. 3T3 cells were transfected with a marker for filamentous actin (f-tractin, green) and the N-BAR domain of Nadrin2 (left, red) or Amphiphysin1 (right, red), respectively. (e) N-BAR domain and a PM marker only partially overlap. 3T3 cells transfected with a membrane marker (CAAX, green) and the N-BAR domain of Nadrin2 (left, red) and Amphiphysin1 (right, red) are shown. (f) Addition of the actin polymerization inhibitor LatA reversibly inhibits N-BAR domain puncta formation. 1303 individual puncta from 12 cells were analyzed for the drug washout experiments. (g) Addition of the actin polymerization inhibitor CytoD reversibly inhibits N-BAR domain puncta formation. 428 puncta from 8 cells were analyzed for the drug washout experiments. Scale bars (a-g), 10μm. Error bars represent s.e.m. of the mean value. ** P < 0.01.
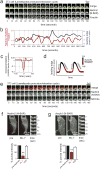
Figure 4. MyosinII-dependent contraction of actin cables induces N-BAR domain recruitment to the lamellipodia PM
(a) N-BAR domain of Nadrin2 recruits to contracting lamellipodia. Montage depicting expansion/collapse cycle in 3T3 cells lamellipodia. Merged image of N-BAR domain of Nadrin2 (red) and f-tractin (green) at an interval of 10 seconds are shown in the top row. For clarification, separate channels of the fluorescently tagged N-BAR domain of Nadrin2 (middle row) and f-tractin (bottom row) are shown below. Note the delayed accumulation of Nadrin2 during the retractive phase of lamellipodia. (b) Comparison of normalized N-BAR domain intensity during expansion/collapse cycle of the lamellipodia. Correlation of the N-BAR domain concentration normalized to f-tractin (red) and lamellipodia position (blue) are show for an individual position on the leading edge monitored over an interval of 1400 seconds. (c) Fluorescent level of N-BAR domain (after normalization to f-tractin intensity) anti-correlate with expansion of the leading edge. Graph depicting average cross-correlation of normalized N-BAR concentration at the leading edge with the position of the leading edge over time. (d) Model of the normalized N-BAR domain of Nadrin2 concentration as a function of the leading edge position. (e) MHC2a and the N-BAR domain of Nadrin2 both enrich during lamellipodia retraction, N-BAR close to the front and MyosinII a bit further back. Montage of a 3T3 cell expressing Nadrin2 (red) and MHC2a (green) shows accumulation of both proteins during lamella retraction. (f, g) Inhibition of MyosinII causes rapid disassembly of N-BAR domain puncta at the leading edge of 3T3 cells. Addition of the MLCK inhibitor ML-7 triggers rapid disassembly of the N-BAR domain of Nadrin2 (f, red, n=8 cells) and Amphiphysin1 (g, red, n=12 cells) puncta, respectively. In contrast, no effect was visible upon addition of water (white, n=6 cells). For better visualization, a montage of a magnified section is depicted next to the pictures. Scale bars (a, e), 5 μm; (f, g), 10 μm. Error bars represent s.e.m. of the mean value, ** P < 0.01.
Figure 5. External push and internal pull forces applied to local PM sites recruit N-BAR domains to inward curved membrane
(a) Scanning Electron micrographs depicting membrane deformation at the leading edge. For better visualization, a magnification of a selected region (white box, bottom) depicting a lamellipodium is shown (top). (b) TEM of 3T3 cell parallel to the glass plane indicating actin filaments pulling at the PM as a source of plasma deformation. For better visualization the ends of individual actin cables are highlighted in red. (c) N-BAR domain of Nadrin2 accumulates on membrane deformations at the leading edge. 3T3 cells were transfected with fluorescently tagged N-BAR domain of Nadrin2, fixed and incubated with primary antibody directed against the fluorescent tag. Enrichment of Immunogold particles to curved sites was measured (n= 15 cells) and compared to adjacent regions within the same image. Note that gold-conjugated secondary antibody is significantly enriched on inward curved membrane sites (graph, bottom). (d) Different types of force-dependent membrane deformations recruit N-BAR domain proteins. N-BAR domain proteins (red) sense positive (inward) membrane curvature induced by external force such as the Nanocone substrate (left), during MyosinII triggered actin contraction (middle), and during endocytosis (right). Error bars represent s.e.m. of the mean value; ** P < 0.01.
Similar articles
- Nanocones to study initial steps of endocytosis.
Jeong S, Galic M. Jeong S, et al. Methods Mol Biol. 2014;1174:275-84. doi: 10.1007/978-1-4939-0944-5_19. Methods Mol Biol. 2014. PMID: 24947389 - BAR domains as sensors of membrane curvature: the amphiphysin BAR structure.
Peter BJ, Kent HM, Mills IG, Vallis Y, Butler PJ, Evans PR, McMahon HT. Peter BJ, et al. Science. 2004 Jan 23;303(5657):495-9. doi: 10.1126/science.1092586. Epub 2003 Nov 26. Science. 2004. PMID: 14645856 - I-BAR domain proteins: linking actin and plasma membrane dynamics.
Zhao H, Pykäläinen A, Lappalainen P. Zhao H, et al. Curr Opin Cell Biol. 2011 Feb;23(1):14-21. doi: 10.1016/j.ceb.2010.10.005. Epub 2010 Nov 17. Curr Opin Cell Biol. 2011. PMID: 21093245 - BAR, F-BAR (EFC) and ENTH/ANTH domains in the regulation of membrane-cytosol interfaces and membrane curvature.
Itoh T, De Camilli P. Itoh T, et al. Biochim Biophys Acta. 2006 Aug;1761(8):897-912. doi: 10.1016/j.bbalip.2006.06.015. Epub 2006 Jul 28. Biochim Biophys Acta. 2006. PMID: 16938488 Review. - Higher-order assemblies of BAR domain proteins for shaping membranes.
Suetsugu S. Suetsugu S. Microscopy (Oxf). 2016 Jun;65(3):201-10. doi: 10.1093/jmicro/dfw002. Epub 2016 Feb 15. Microscopy (Oxf). 2016. PMID: 26884618 Review.
Cited by
- Opportunities and dilemmas of in vitro nano neural electrodes.
Wu Y, Chen H, Guo L. Wu Y, et al. RSC Adv. 2019 Dec 24;10(1):187-200. doi: 10.1039/c9ra08917a. eCollection 2019 Dec 20. RSC Adv. 2019. PMID: 35492533 Free PMC article. Review. - Sensing their plasma membrane curvature allows migrating cells to circumvent obstacles.
Sitarska E, Almeida SD, Beckwith MS, Stopp J, Czuchnowski J, Siggel M, Roessner R, Tschanz A, Ejsing C, Schwab Y, Kosinski J, Sixt M, Kreshuk A, Erzberger A, Diz-Muñoz A. Sitarska E, et al. Nat Commun. 2023 Sep 13;14(1):5644. doi: 10.1038/s41467-023-41173-1. Nat Commun. 2023. PMID: 37704612 Free PMC article. - Geometric protein localization cues in bacterial cells.
Updegrove TB, Ramamurthi KS. Updegrove TB, et al. Curr Opin Microbiol. 2017 Apr;36:7-13. doi: 10.1016/j.mib.2016.12.001. Epub 2017 Jan 19. Curr Opin Microbiol. 2017. PMID: 28110195 Free PMC article. Review. - Cellular nanointerface of vertical nanostructure arrays and its applications.
Zhang A, Fang J, Li X, Wang J, Chen M, Chen HJ, He G, Xie X. Zhang A, et al. Nanoscale Adv. 2022 Feb 21;4(8):1844-1867. doi: 10.1039/d1na00775k. eCollection 2022 Apr 12. Nanoscale Adv. 2022. PMID: 36133409 Free PMC article. Review. - Molecular Dynamics Simulations of Curved Lipid Membranes.
Larsen AH. Larsen AH. Int J Mol Sci. 2022 Jul 22;23(15):8098. doi: 10.3390/ijms23158098. Int J Mol Sci. 2022. PMID: 35897670 Free PMC article. Review.
References
- Suetsugu S, Toyooka K, Senju Y. Subcellular membrane curvature mediated by the BAR domain superfamily proteins. Semin Cell Dev Biol. 2010;21:340–349. S1084-9521 (09)00247-X [pii] 10.1016/j.semcdb.2009.12.002. - PubMed
- Ringstad N, et al. Endophilin/SH3p4 is required for the transition from early to late stages in clathrin-mediated synaptic vesicle endocytosis. Neuron. 1999;24:143–154. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 MH064801/MH/NIMH NIH HHS/United States
- MH064801/MH/NIMH NIH HHS/United States
- R01 GM063702/GM/NIGMS NIH HHS/United States
- R01 MH095087/MH/NIMH NIH HHS/United States
- R01 GM030179/GM/NIGMS NIH HHS/United States
- GM063702/GM/NIGMS NIH HHS/United States
- MH095087/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous