Soft fibrin gels promote selection and growth of tumorigenic cells - PubMed (original) (raw)
Soft fibrin gels promote selection and growth of tumorigenic cells
Jing Liu et al. Nat Mater. 2012.
Erratum in
- Author Correction: Soft fibrin gels promote selection and growth of tumorigenic cells.
Liu J, Tan Y, Zhang H, Zhang Y, Xu P, Chen J, Poh YC, Tang K, Wang N, Huang B. Liu J, et al. Nat Mater. 2021 Jun;20(6):905. doi: 10.1038/s41563-021-01032-0. Nat Mater. 2021. PMID: 34007051 No abstract available.
Abstract
The identification of stem-cell-like cancer cells through conventional methods that depend on stem cell markers is often unreliable. We developed a mechanical method for selecting tumorigenic cells by culturing single cancer cells in fibrin matrices of ~100 Pa in stiffness. When cultured within these gels, primary human cancer cells or single cancer cells from mouse or human cancer cell lines grew within a few days into individual round colonies that resembled embryonic stem cell colonies. Subcutaneous or intravenous injection of 10 or 100 fibrin-cultured cells in syngeneic or severe combined immunodeficiency mice led to the formation of solid tumours at the site of injection or at the distant lung organ much more efficiently than control cancer cells selected using conventional surface marker methods or cultured on conventional rigid dishes or on soft gels. Remarkably, as few as ten such cells were able to survive and form tumours in the lungs of wild-type non-syngeneic mice.
Figures
Fig. 1. Multicellular tumour spheroid formation in soft 3D fibrin gels
(a) A single B16-F1 cell grew into a multicellular tumour spheroid within 90-Pa 3D fibrin gel during culture course from Day 1 to Day 5. (b) Multicellular B16-F1 tumour spheroid formation after 5 days in culture within soft 3-D fibrin gels of different stiffness. (c) Multicellular tumour spheroid (round colony) number as a function of culture time: Day 1 to Day 6. The 90-Pa fibrin gel appears to be optimal for sustaining spheroid colony number. Mean ± s.e.m., n=6 (for 90-Pa gels) or 3 (for 420-Pa or 1050-Pa gels) independent experiments. There are no significant differences between 90-Pa and 420- Pa gels at Day 1 (_p_>0.2); there are significant differences at Day 2 through Day 6 (all p<0.022). There are significant differences between 90-Pa and 1050-Pa at Day 1 through Day 6 (all p<0.018). Between 420-Pa and 1050-Pa gels, there are differences only at Day 1 (_p_=0.037) and at Day 5 (_p_=0.00082). (d) Colony size of multicellular tumour spheroid as a function of culture time and fibrin stiffness. Apparently 90-Pa fibrin best promotes tumour growth. Mean ± s.e.m.; n=6 (for 90-Pa gels) or 3 (for 420-Pa or 1050-Pa gels) independent experiments. The stiffness of 3D fibrin gels with concentrations of 1 mg/ml, 4 mg/ml and 8 mg/ml is 90, 420 and 1050 Pa, respectively. There are no significant differences at Day 1 between 90 Pa and 420 Pa (_p_=0.27) or between 90-Pa and 1050-Pa gels (_p_=0.33); from Day 2 through Day 6, there are significant differences between 90-Pa and 420-Pa (p<0.002 for all paired comparisons) and between 90 Pa and 1050 Pa gels (all p<0.0012). The data in (c) and (d) are fitted by the 3rd order polynomial functions (solid lines), the parameters of which are given in the Methods section.
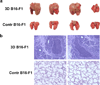
Fig. 2. Tumour metastasis of 3D cultured B16-F1 cells in lung tissue of BALB/c mice
(a) B16-F1 tumour spheroids formed in soft 3D fibrin gel after 5-day culture were injected into tail vein of BALB/c mice with 10 cells. Mice were sacrificed after 2 months of injection and lung tissue image was recorded. B16-F1 cells cultured in 2D rigid dish were used as control. The results shown were a representative from three independent experiments. (b) Lung tissues of the above B16-F1 tumour spheroid treated BALB/c mice were analyzed by H&E staining. B16-F1 cells cultured on 2D rigid dish were used as control.
Fig. 3. Upregulation of stem cell-associated genes in B16-F1 spheroid cells cultured in 3D fibrin gel
(a) Stem cell marker expression in B16-F1 spheroid cells. Total mRNA of B16-F1 spheroid cells at Day 5 was extracted and used for the detection of Nanog, Oct3/4, CD133, nestin, Bmi-1 and c-kit mRNA expression by RT-PCR. B16-F1 cells cultured in 2D rigid dish were used as control. Three independent experiments showed similar results. (b) Murine telomerase reverse transcriptase subunit (mTERT) expression of B16-F1 spheroid cells. mTERT mRNA expression was measured by RT-PCR; representative images of 3 independent experiments. (c) and (d) Stem cells markers and mTERT expression in B16-F1 cells are quantified by real-time PCR. Same mRNA sample of B16-F1 tumour spheroid cells was used as above. B16-F1 cells cultured in 2D rigid dish were used as control. Mean ± s.e.m., *p <0.05, compared with control cells. (e) Apoptotic analysis of doxorubicin-treated 3D B16-F1 cells. Different concentrations of doxorubicin were added to the B16-F1 cell culture medium during the last 18 hours of 5-day culture in the 90-Pa 3D fibrin gels or conventional 2D rigid dish. Cells were collected and stained with FITC conjugated Annexin-V for apoptotic detection by flow cytometry. Mean ± s.e.m..; n=3 independent experiments; *p <0.05, compared with control cells.
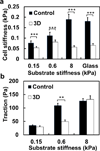
Fig. 4. 3D fibrin-gel cultured B16-F1 cells do not stiffen but elevate tractions with substrate rigidity
(a) Cell stiffness (shear modulus) as a function of substrate stiffness. 3D fibrin gel cultured B16-F1 cells do not stiffen much with substrate rigidity, whereas control cancer cells do. Mean ± s.e.m., 5 independent experiments; at least 150 cells per experiment. (b) Both 3D fibrin gel cultured B16-F1 cells and control cells elevate tractions on stiffer substrates. The cells were plated for 6 hr before experiments in both (a) and (b). Mean ± s.e.m.; 3 independent experiments, n> 60 cells for each value (**, p<0.01; ***, p<0.001.)
Comment in
- Cell culture: Soft gels select tumorigenic cells.
Shin JW, Discher DE. Shin JW, et al. Nat Mater. 2012 Jul 24;11(8):662-3. doi: 10.1038/nmat3388. Nat Mater. 2012. PMID: 22825018 No abstract available. - The dark side of scaffolds.
Sachlos E, Bollenbach T, Kerstetter-Fogle AE, King CC. Sachlos E, et al. Regen Med. 2013 Jan;8(1):17. doi: 10.2217/rme.12.111. Regen Med. 2013. PMID: 23259801 No abstract available.
Similar articles
- Cell culture: Soft gels select tumorigenic cells.
Shin JW, Discher DE. Shin JW, et al. Nat Mater. 2012 Jul 24;11(8):662-3. doi: 10.1038/nmat3388. Nat Mater. 2012. PMID: 22825018 No abstract available. - The dark side of scaffolds.
Sachlos E, Bollenbach T, Kerstetter-Fogle AE, King CC. Sachlos E, et al. Regen Med. 2013 Jan;8(1):17. doi: 10.2217/rme.12.111. Regen Med. 2013. PMID: 23259801 No abstract available. - Soft fibrin matrix downregulates DAB2IP to promote Nanog-dependent growth of colon tumor-repopulating cells.
Zhang M, Xu C, Wang HZ, Peng YN, Li HO, Zhou YJ, Liu S, Wang F, Liu L, Chang Y, Zhao Q, Liu J. Zhang M, et al. Cell Death Dis. 2019 Feb 15;10(3):151. doi: 10.1038/s41419-019-1309-7. Cell Death Dis. 2019. PMID: 30770783 Free PMC article. - Association between tumorigenic potential and the fate of cancer cells in a syngeneic melanoma model.
Krelin Y, Berkovich L, Amit M, Gil Z. Krelin Y, et al. PLoS One. 2013 Apr 23;8(4):e62124. doi: 10.1371/journal.pone.0062124. Print 2013. PLoS One. 2013. PMID: 23626777 Free PMC article. - Metastases of human tumors in experimental animals.
Doré JF, Bailly M, Bertrand S. Doré JF, et al. Anticancer Res. 1987 Sep-Oct;7(5B):997-1003. Anticancer Res. 1987. PMID: 3324940 Review.
Cited by
- Fibronectin Matrix Formation is a Prerequisite for Colonization of Kidney Tumor Cells in Fibrin.
Knowles LM, Gurski LA, Maranchie JK, Pilch J. Knowles LM, et al. J Cancer. 2015 Jan 1;6(2):98-104. doi: 10.7150/jca.10496. eCollection 2015. J Cancer. 2015. PMID: 25561973 Free PMC article. - A pH-responsive Pickering Nanoemulsion for specified spatial delivery of Immune Checkpoint Inhibitor and Chemotherapy agent to Tumors.
Jia L, Pang M, Fan M, Tan X, Wang Y, Huang M, Liu Y, Wang Q, Zhu Y, Yang X. Jia L, et al. Theranostics. 2020 Aug 7;10(22):9956-9969. doi: 10.7150/thno.46089. eCollection 2020. Theranostics. 2020. PMID: 32929327 Free PMC article. - Aryl hydrocarbon receptor sulfenylation promotes glycogenolysis and rescues cancer chemoresistance.
Zhou N, Chen J, Ling Z, Zhang C, Zhou Y, Wang D, Zhou L, Wang Z, Sun N, Wang X, Zhang H, Tang K, Ma J, Lv J, Huang B. Zhou N, et al. J Clin Invest. 2023 Dec 15;133(24):e170753. doi: 10.1172/JCI170753. J Clin Invest. 2023. PMID: 38099490 Free PMC article. - Tuning Enzymatically Crosslinked Silk Fibroin Hydrogel Properties for the Development of a Colorectal Cancer Extravasation 3D Model on a Chip.
Carvalho MR, Maia FR, Vieira S, Reis RL, Oliveira JM. Carvalho MR, et al. Glob Chall. 2018 May 24;2(5-6):1700100. doi: 10.1002/gch2.201700100. eCollection 2018 Jun 26. Glob Chall. 2018. PMID: 31565332 Free PMC article. - The Role of Extracellular Matrix Remodeling in Skin Tumor Progression and Therapeutic Resistance.
Fromme JE, Zigrino P. Fromme JE, et al. Front Mol Biosci. 2022 Apr 26;9:864302. doi: 10.3389/fmolb.2022.864302. eCollection 2022. Front Mol Biosci. 2022. PMID: 35558554 Free PMC article. Review.
References
- Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat. Med. 1997;3:730–737. - PubMed
- Civenni G, et al. Human CD271-positive melanoma stem cells associated with metastasis establish tumour heterogeneity and long-term growth. Cancer Res. 2011;71:3098–3109. - PubMed
- Gupta PB, Chaffer CL, Weinberg RA. Cancer stem cells: mirage or reality? Nat. Med. 2009;15:1010–1012. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources