Transcriptomes of germinal zones of human and mouse fetal neocortex suggest a role of extracellular matrix in progenitor self-renewal - PubMed (original) (raw)
. 2012 Jul 17;109(29):11836-41.
doi: 10.1073/pnas.1209647109. Epub 2012 Jul 2.
Robert Lachmann, Holger Brandl, Martin Kircher, Nikolay Samusik, Roland Schröder, Naharajan Lakshmanaperumal, Ian Henry, Johannes Vogt, Axel Riehn, Wolfgang Distler, Robert Nitsch, Wolfgang Enard, Svante Pääbo, Wieland B Huttner
Affiliations
- PMID: 22753484
- PMCID: PMC3406833
- DOI: 10.1073/pnas.1209647109
Transcriptomes of germinal zones of human and mouse fetal neocortex suggest a role of extracellular matrix in progenitor self-renewal
Simone A Fietz et al. Proc Natl Acad Sci U S A. 2012.
Abstract
The expansion of the neocortex during mammalian brain evolution results primarily from an increase in neural progenitor cell divisions in its two principal germinal zones during development, the ventricular zone (VZ) and the subventricular zone (SVZ). Using mRNA sequencing, we analyzed the transcriptomes of fetal human and embryonic mouse VZ, SVZ, and cortical plate. In mouse, the transcriptome of the SVZ was more similar to that of the cortical plate than that of the VZ, whereas in human the opposite was the case, with the inner and outer SVZ being highly related to each other despite their cytoarchitectonic differences. We describe sets of genes that are up- or down-regulated in each germinal zone. These data suggest that cell adhesion and cell-extracellular matrix interactions promote the proliferation and self-renewal of neural progenitors in the developing human neocortex. Notably, relevant extracellular matrix-associated genes include distinct sets of collagens, laminins, proteoglycans, and integrins, along with specific sets of growth factors and morphogens. Our data establish a basis for identifying novel cell-type markers and open up avenues to unravel the molecular basis of neocortex expansion during evolution.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
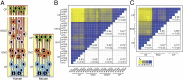
Fig. 1.
Correlation analysis of fetal human and embryonic mouse cortical zones. (A) Cartoon illustrating the major cell types (APs in blue, BPs in orange and red, neurons in green) in the different GZs and the CP of the developing mouse and human neocortex. (B and C) Heat-map showing pair-wise correlations of gene expression levels between the indicated zones of the fetal human neocortex at the indicated gestational weeks (B, 13–16 wpc) and of the E14.5 mouse neocortex (C). Spearman’s rank correlation coefficients (_r_s) range from 0.7 (yellow) to 1.0 (blue). Numbers in the blank quadrants indicate the mean _r_s values ± SD of the zone comparison located mirror-symmetrically to the diagonal line; asterisks indicate zone comparison mean _r_s values that show a statistically significant difference to each other (P < 0.05).
Fig. 2.
Differential gene-expression analysis and functional annotation clustering of fetal human cortical zones. (A) DESeq scatter plot showing pair-wise comparisons of gene expression between the indicated zones of the 14–16 wpc human neocortex. Each dot represents the mean (five fetuses) expression level for a given gene, with differentially expressed genes (false-discovery rate <0.05) shown in green. Numbers above (below) the diagonal lines refer to the differentially expressed genes up-regulated in the zone indicated on top (on the right). These numbers include the genes expressed in only one of the two zones compared (green dots on vertical and horizontal 0.1 lines). For the corresponding analysis of genes differentially expressed in mouse zones and between human and mouse zones see
Fig. S5
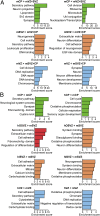
. (B) Venn diagrams showing the numbers of differentially expressed genes (see A) that are up-regulated (Left, >) and down-regulated (Right, <) in a given zone compared with the other zones of the 14–16 wpc human neocortex. (_C_) Differentially expressed genes (see _A_) that are up-regulated (_Left_, >) and down-regulated (Right, <) in a given zone compared with the other zones indicated of the 14–16 wpc human neocortex were analyzed for significantly enriched FGA terms which were clustered, using DAVID. The five clusters with the highest enrichment scores are shown. Note that for certain FGA terms enriched in the various GZs, the same designation were chosen (e.g., secretory pathway), although the individual genes in these clusters were different.
Fig. 3.
Functional annotation clustering of genes differentially expressed among mouse cortical zones and between human and mouse cortical zones. Differentially expressed genes identified by DESeq (
Fig. S5
) that are up-regulated (Left, >) and down-regulated (Right, <) between the indicated zones of the E14.5 mouse neocortex (A) and between the 14–16 wpc human and E14.5 mouse neocortex (B) were analyzed for significantly enriched FGA terms, which were clustered, using DAVID. The five clusters with the highest enrichment scores are shown. See note on FGA terms in Fig. 2_C_.
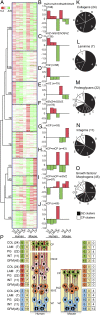
Fig. 4.
Mean-shift clustering of genes related to ECM interaction in fetal human and embryonic mouse cortical zones. (A–J) Log2-transformed FPKM fold-changes of ECM-related genes in the 14–16 wpc hVZ, hISVZ, hOSVZ, and hCP (five fetuses) and in the E14.5 mVZ, mSVZ, and mCP (five embryos) relative to the mean expression of each of the genes in all human or mouse zones were subjected to nonhierarchical K-nearest neighbor mean-shift (KNN-MS) cluster analysis, which yielded a total of nine clusters. (A) Heat map showing the nine clusters sorted hierarchically using an average-linkage algorithm. Each gene is represented by a single row and each developmental stage by a single column. Fold-changes range from 5.2 (red) to −6.6 (green). (B–J) Median log2 fold-changes in expression of the genes (numbers in parentheses) contained in the nine clusters shown in A. (K–O) Occurrence of collagens (K), laminins (L), proteoglycans (M), integrins (N), and growth factors/morphogens (O) in the clusters indicated in B–J of the 14–16 wpc human (five fetuses) or E14.5 mouse (five embryos) neocortex. Numbers in parentheses refer to the total number of collagen, laminin, proteoglycan, integrin, or growth factor/morphogen genes expressed in the fetal human and embryonic mouse neocortex; numbers in the pie chart sectors refer to the number of the respective genes expressed in the indicated cluster. Black sectors, GZ clusters; white sectors, CP clusters. (P) Summary of the distribution of collagens (COL), laminins (LAM), proteoglycans (PG), integrins (INT), and growth factors/morphogens (GFM) between the various zones of fetal human and embryonic mouse neocortex (APs in blue, BPs in orange and red, neurons in green). Numbers in parentheses, total number of genes in respective class, as indicated in K–O; numbers in green/red/blue boxes, number of genes in respective class that are specifically overexpressed (red bars in B–J) only in the hCP or mCP (green), hISVZ, and hOSVZ or mSVZ (red), or hVZ or mVZ (blue); numbers in yellow boxes, number of genes in respective class that are overexpressed in more than one human or mouse zone; numbers in white boxes, number of genes in respective class that are overexpressed in both h/mVZ, hISVZ/hOSVZ/mSVZ, or h/mCP.
Similar articles
- Oblique radial glial divisions in the developing mouse neocortex induce self-renewing progenitors outside the germinal zone that resemble primate outer subventricular zone progenitors.
Shitamukai A, Konno D, Matsuzaki F. Shitamukai A, et al. J Neurosci. 2011 Mar 9;31(10):3683-95. doi: 10.1523/JNEUROSCI.4773-10.2011. J Neurosci. 2011. PMID: 21389223 Free PMC article. - Laminin regulates postnatal oligodendrocyte production by promoting oligodendrocyte progenitor survival in the subventricular zone.
Relucio J, Menezes MJ, Miyagoe-Suzuki Y, Takeda S, Colognato H. Relucio J, et al. Glia. 2012 Oct;60(10):1451-67. doi: 10.1002/glia.22365. Epub 2012 Jun 17. Glia. 2012. PMID: 22706957 Free PMC article. - Spatial transcriptome reveals the region-specific genes and pathways regulated by Satb2 in neocortical development.
Yang J, Li Y, Tang Y, Yang L, Guo C, Peng C. Yang J, et al. BMC Genomics. 2024 Aug 2;25(1):757. doi: 10.1186/s12864-024-10672-w. BMC Genomics. 2024. PMID: 39095712 Free PMC article. - Cytoarchitecture of mouse and human subventricular zone in developing cerebral neocortex.
Tabata H, Yoshinaga S, Nakajima K. Tabata H, et al. Exp Brain Res. 2012 Jan;216(2):161-8. doi: 10.1007/s00221-011-2933-3. Epub 2011 Nov 13. Exp Brain Res. 2012. PMID: 22080150 Free PMC article. Review. - The Subventricular Zone: A Key Player in Human Neocortical Development.
Ortega JA, Memi F, Radonjic N, Filipovic R, Bagasrawala I, Zecevic N, Jakovcevski I. Ortega JA, et al. Neuroscientist. 2018 Apr;24(2):156-170. doi: 10.1177/1073858417691009. Epub 2017 Feb 13. Neuroscientist. 2018. PMID: 29254416 Review.
Cited by
- Radial glia progenitor polarity in health and disease.
Viola V, Chinnappa K, Francis F. Viola V, et al. Front Cell Dev Biol. 2024 Oct 2;12:1478283. doi: 10.3389/fcell.2024.1478283. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 39416687 Free PMC article. Review. - Gene expansions contributing to human brain evolution.
Soto DC, Uribe-Salazar JM, Kaya G, Valdarrago R, Sekar A, Haghani NK, Hino K, La GN, Mariano NAF, Ingamells C, Baraban AE, Turner TN, Green ED, Simó S, Quon G, Andrés AM, Dennis MY. Soto DC, et al. bioRxiv [Preprint]. 2024 Sep 26:2024.09.26.615256. doi: 10.1101/2024.09.26.615256. bioRxiv. 2024. PMID: 39386494 Free PMC article. Preprint. - A multi-layered integrative analysis reveals a cholesterol metabolic program in outer radial glia with implications for human brain evolution.
Moriano J, Leonardi O, Vitriolo A, Testa G, Boeckx C. Moriano J, et al. Development. 2024 Aug 15;151(16):dev202390. doi: 10.1242/dev.202390. Epub 2024 Aug 27. Development. 2024. PMID: 39114968 Free PMC article. - Dysregulation of FLVCR1a-dependent mitochondrial calcium handling in neural progenitors causes congenital hydrocephalus.
Bertino F, Mukherjee D, Bonora M, Bagowski C, Nardelli J, Metani L, Zanin Venturini DI, Chianese D, Santander N, Salaroglio IC, Hentschel A, Quarta E, Genova T, McKinney AA, Allocco AL, Fiorito V, Petrillo S, Ammirata G, De Giorgio F, Dennis E, Allington G, Maier F, Shoukier M, Gloning KP, Munaron L, Mussano F, Salsano E, Pareyson D, di Rocco M, Altruda F, Panagiotakos G, Kahle KT, Gressens P, Riganti C, Pinton PP, Roos A, Arnold T, Tolosano E, Chiabrando D. Bertino F, et al. Cell Rep Med. 2024 Jul 16;5(7):101647. doi: 10.1016/j.xcrm.2024.101647. Cell Rep Med. 2024. PMID: 39019006 Free PMC article. - Indirect neurogenesis in space and time.
Thor S. Thor S. Nat Rev Neurosci. 2024 Aug;25(8):519-534. doi: 10.1038/s41583-024-00833-x. Epub 2024 Jul 1. Nat Rev Neurosci. 2024. PMID: 38951687 Review.
References
- Fietz SA, Huttner WB. Cortical progenitor expansion, self-renewal and neurogenesis-a polarized perspective. Curr Opin Neurobiol. 2011;21:23–35. - PubMed
- Molnár Z. Evolution of cerebral cortical development. Brain Behav Evol. 2011;78:94–107. - PubMed
- Götz M, Huttner WB. The cell biology of neurogenesis. Nat Rev Mol Cell Biol. 2005;6:777–788. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical