A dual-tracer method for differentiating transendothelial transport from paracellular leakage in vivo and in vitro - PubMed (original) (raw)
A dual-tracer method for differentiating transendothelial transport from paracellular leakage in vivo and in vitro
Nino Muradashvili et al. Front Physiol. 2012.
Abstract
Inflammation-induced impaired function of vascular endothelium may cause leakage of plasma proteins that can lead to edema. Proteins may leave the vascular lumen through two main paracellular and transcellular pathways. As the first involves endothelial cell (EC) junction proteins and the second caveolae formation, these two pathways are interconnected. Therefore, it is difficult to differentiate the prevailing role of one or the other pathway during pathology that causes inflammation. Here we present a newly developed dual-tracer probing method that allows differentiation of transcellular from paracellular transport during pathology. This fluorescence-based method can be used in vitro to test changes in EC layer permeability and in vivo in various animal vascular preparations. The method is based on comparison of low molecular weight molecule (LMWM) transport to that of high molecular weight molecule (HMWM) transport through the EC layer or the vascular wall during physiological and pathological conditions. Since the LMWM will leak through mainly the paracellular and HMWM will move through paracellular (when gaps between the ECs are wide enough) and transcellular pathways, the difference in transport rate (during normal conditions and pathology) of these molecules will indicate the prevailing transport pathway involved in overall protein crossing of vascular wall. Thus, the novel approach of assessing the transport kinetics of different size tracers in vivo by intravital microscopy can clarify questions related to identification of target pathways for drug delivery during various pathologies associated with elevated microvascular permeability.
Keywords: cerebrovascular leakage; fluorescent dyes; intravital microscopy.
Figures
Figure 1
Genotyping of hyperfibrinogenic (HFg) – fibrinogen transgene positive and wild type (WT) mice. Single PCR products suggest the homozygous mutation (HFg), while its absence represents WT allele.
Figure 2
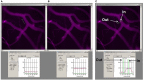
Image analysis of microvascular leakage using Image-Pro Plus software. Examples of pial microvascular bed images (with circulating BSA-647) with a digital Line Profile probe (LPP) placed in the area of interest (upper row) and corresponding results of fluorescent light intensity readings shown in respective Line profile boxes (lower row). About thirty micrometer long LPP was first placed in interstitium adjacent to the venular segment (A) and then inside the venular segment (B). To perform quantitative analysis of fluorescence intensity along a LPP the following steps were followed: , (this led to appearance of the Line profile box. The size and the place of the LPP on the image were adjusted manually), in the Line profile box, then “statistics.” For assessment of fluorescence intensity of BSA-647 was chosen in the Line profile box, while was chosen for FITC intensity measurement. Values of the fluorescence intensities were given as “Mean” and “Standard deviation” in the Line profile box (see lower row). Fluorescence intensity profile along the LPP for each image is shown in the Line profile box below the respective image. Mean values averaged along the LPP are also shown in the Line profile box. Note that mean fluorescence intensity inside the venule is 92.3 fluorescence intensity units (FIU) and in the adjacent interstitium is 26.6 FIU. Thus ratio of fluorescence intensity in interstitium to that in the venule is 0.29. This value was expressed as a percent of baseline values calculated for images obtained immediately after infusion of the dual-tracer probe. Example of the LPP placed across the venular segment and over the adjacent interstitium (upper image) with the fluorescence intensity profile along the LPP (below) is shown (C). Arrows indicate precise locations of where the fluorescence intensities were measured along the LPP. Note that fluorescence intensity inside the venule (In) is 85.1 FIU and in interstitium (Out) is 23. Thus ratio of fluorescence intensity in interstitium to that in the venule in the marked places along the LPP is 0.27. However, to obtain reliable data the LPP should be moved along the vessel and several measurements performed to calculate an average of these results. Since the same data, with better precision, can be obtained using the method described above (A,B), we placed the LPP across the vessel to define the vascular wall and the distance from the vessel where the LPP should have been placed (parallel to the vessel) in interstitium.
Figure 3
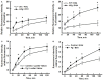
Differentiation of transcellular from paracellular transport through microvascular wall and endothelial cell (EC) layer using a dual-tracer probing method. Leakage of fluorescein isothiocyanate (FITC; upper left panel) or Lucifer Yellow (bottom left panel) and Alexa Fluor 647-conjugated bovine serum albumin BSA (BSA-647; right column) was studied in pial venules of wild type (WT) and hyperfibrinogenic (HFg) mice (A,B), and in mouse brain ECs (MBECs) treated with medium alone (control) or with 4 mg/ml of Fg in medium (Fg) (C,D). Leakage of FITC was greater in HFg mice at 20 and 40 min after infusion of the dye but at 60 and 120 min the difference was no longer observed (A). However, leakage of BSA-647 was more in HFg mice after 20 min of dye infusion (B). These results indicate that inflammatory changes caused by HFg mediate a transient opening of the EC junction gaps after infusion of the dye causing the leakage of FITC. However, when the gaps are no longer opened more in HFg mice than in WT mice, enhanced caveolae formation and their function still maintains greater leakage of BSA via transcellular transport. Similarly, the leakage of Lucifer Yellow was greater through MBECs treated with 4 mg/ml of Fg at 20 and 40 min after addition of the dye but at 60 and 120 min the difference was no longer observed (C). However, leakage of BSA-647 was more through the MBECs treated with Fg after 20 min of addition of dyes (D). These results confirm the data obtained for mouse pial venules, indicating that HFg affects mainly the transcellular transport through formation of functional caveolae. *p < 0.05 – versus WT or control. n = 6 for animals and n = 8 MBECs.
Similar articles
- Sphingolipids affect fibrinogen-induced caveolar transcytosis and cerebrovascular permeability.
Muradashvili N, Khundmiri SJ, Tyagi R, Gartung A, Dean WL, Lee MJ, Lominadze D. Muradashvili N, et al. Am J Physiol Cell Physiol. 2014 Jul 15;307(2):C169-79. doi: 10.1152/ajpcell.00305.2013. Epub 2014 May 14. Am J Physiol Cell Physiol. 2014. PMID: 24829496 Free PMC article. - Role of caveolin-1 in the regulation of pulmonary endothelial permeability.
Sun Y, Minshall RD, Hu G. Sun Y, et al. Methods Mol Biol. 2011;763:303-17. doi: 10.1007/978-1-61779-191-8_21. Methods Mol Biol. 2011. PMID: 21874461 - Regulation of endothelial permeability by Src kinase signaling: vascular leakage versus transcellular transport of drugs and macromolecules.
Hu G, Place AT, Minshall RD. Hu G, et al. Chem Biol Interact. 2008 Jan 30;171(2):177-89. doi: 10.1016/j.cbi.2007.08.006. Epub 2007 Aug 15. Chem Biol Interact. 2008. PMID: 17897637 Free PMC article. Review. - Co-regulation of transcellular and paracellular leak across microvascular endothelium by dynamin and Rac.
Armstrong SM, Khajoee V, Wang C, Wang T, Tigdi J, Yin J, Kuebler WM, Gillrie M, Davis SP, Ho M, Lee WL. Armstrong SM, et al. Am J Pathol. 2012 Mar;180(3):1308-1323. doi: 10.1016/j.ajpath.2011.12.002. Epub 2011 Dec 25. Am J Pathol. 2012. PMID: 22203054 - Structural pathways for macromolecular and cellular transport across the blood-brain barrier during inflammatory conditions. Review.
Lossinsky AS, Shivers RR. Lossinsky AS, et al. Histol Histopathol. 2004 Apr;19(2):535-64. doi: 10.14670/HH-19.535. Histol Histopathol. 2004. PMID: 15024715 Review.
Cited by
- Developing a transwell millifluidic device for studying blood-brain barrier endothelium.
Harding IC, O'Hare NR, Vigliotti M, Caraballo A, Lee CI, Millican K, Herman IM, Ebong EE. Harding IC, et al. Lab Chip. 2022 Nov 22;22(23):4603-4620. doi: 10.1039/d2lc00657j. Lab Chip. 2022. PMID: 36326069 Free PMC article. - Endothelial dysfunction in preterm infants: The hidden legacy of uteroplacental pathologies.
Amelio GS, Provitera L, Raffaeli G, Tripodi M, Amodeo I, Gulden S, Cortesi V, Manzoni F, Cervellini G, Tomaselli A, Pravatà V, Garrido F, Villamor E, Mosca F, Cavallaro G. Amelio GS, et al. Front Pediatr. 2022 Nov 4;10:1041919. doi: 10.3389/fped.2022.1041919. eCollection 2022. Front Pediatr. 2022. PMID: 36405831 Free PMC article. Review. - Sepsis: network pathophysiology and implications for early diagnosis.
Arora J, Mendelson AA, Fox-Robichaud A. Arora J, et al. Am J Physiol Regul Integr Comp Physiol. 2023 May 1;324(5):R613-R624. doi: 10.1152/ajpregu.00003.2023. Epub 2023 Mar 6. Am J Physiol Regul Integr Comp Physiol. 2023. PMID: 36878489 Free PMC article. Review. - Mechanism of Blood-Heart-Barrier Leakage: Implications for COVID-19 Induced Cardiovascular Injury.
Homme RP, George AK, Singh M, Smolenkova I, Zheng Y, Pushpakumar S, Tyagi SC. Homme RP, et al. Int J Mol Sci. 2021 Dec 17;22(24):13546. doi: 10.3390/ijms222413546. Int J Mol Sci. 2021. PMID: 34948342 Free PMC article. - Cancer Immunotherapy Getting Brainy: Visualizing the Distinctive CNS Metastatic Niche to Illuminate Therapeutic Resistance.
Owyong M, Hosseini-Nassab N, Efe G, Honkala A, van den Bijgaart RJE, Plaks V, Smith BR. Owyong M, et al. Drug Resist Updat. 2017 Nov;33-35:23-35. doi: 10.1016/j.drup.2017.10.001. Epub 2017 Oct 14. Drug Resist Updat. 2017. PMID: 29145972 Free PMC article. Review.
References
- Bauer P. M., Yu J., Chen Y., Hickey R., Bernatchez P. N., Looft-Wilson R., Huang Y., Giordano F., Stan R. V., Sessa W. C. (2005). Endothelial-specific expression of caveolin-1 impairs microvascular permeability and angiogenesis. Proc. Natl. Acad. Sci. U.S.A. 102, 204–20910.1073/pnas.0406092102 - DOI - PMC - PubMed
- Curry F.-R. E., Rygh C. B., Karlsen T., Wiig H., Adamson R. H., Clark J. F., Lin Y.-C., Gassner B., Thorsen F., Moen I., Tenstad O., Kuhn M., Reed R. K. (2010). Atrial natriuretic peptide modulation of albumin clearance and contrast agent permeability in mouse skeletal muscle and skin: role in regulation of plasma volume. J. Physiol. (Lond.) 588, 325–33910.1113/jphysiol.2009.180463 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials