Chelators in the treatment of iron accumulation in Parkinson's disease - PubMed (original) (raw)
Chelators in the treatment of iron accumulation in Parkinson's disease
Ross B Mounsey et al. Int J Cell Biol. 2012.
Abstract
Iron is an essential element in the metabolism of all cells. Elevated levels of the metal have been found in the brains of patients of numerous neurodegenerative disorders, including Parkinson's disease (PD). The pathogenesis of PD is largely unknown, although it is thought through studies with experimental models that oxidative stress and dysfunction of brain iron homeostasis, usually a tightly regulated process, play significant roles in the death of dopaminergic neurons. Accumulation of iron is present at affected neurons and associated microglia in the substantia nigra of PD patients. This additional free-iron has the capacity to generate reactive oxygen species, promote the aggregation of α-synuclein protein, and exacerbate or even cause neurodegeneration. There are various treatments aimed at reversing this pathologic increase in iron content, comprising both synthetic and natural iron chelators. These include established drugs, which have been used to treat other disorders related to iron accumulation. This paper will discuss how iron dysregulation occurs and the link between increased iron and oxidative stress in PD, including the mechanism by which these processes lead to cell death, before assessing the current pharmacotherapies aimed at restoring normal iron redox and new chelation strategies undergoing research.
Figures
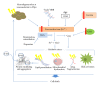
Figure 1
Iron-mediated cell death in PD. Reduced storage capacity in PD due to decreased ferritin expression and degeneration of nigral melatonin-containing neurons causes an increase in the reactive Fe2+ iron pool. Age-related increases in iron and a leaky BBB cause further iron accumulation. The transfer of the free iron to ferric iron, Fe3+, in the hydrogen peroxide-mediated Fenton reaction produces the highly toxic hydroxyl radical. A compromised level of glutathione exacerbates the levels of free radicals, whilst the deamination and autoxidation of dopamine produces further H2O2. The subsequent oxidative stress can then elicit a range of cytotoxic reactions including protein misfolding, lipid peroxidation (which, in turn, can cause _α_-synuclein aggregation), mitochondrial dysfunction, and activation of glial cells. These various insults can induce cell death by apoptosis, causing further degeneration.
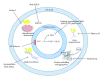
Figure 2
Action of iron chelators targeting PD. All iron chelators mop up excess free, reactive iron, thus reducing the reduction of Fe2+ to Fe3+—a reaction that produces various ROS, such as the hydroxyl radical. Oxidative stress resulting from the generation of ROS produces a range of deleterious insults, which can be targeted with the multiple actions of inhibitors. This can attenuate the cell death that these events induce. Chelators with antioxidant properties inhibit the production of ROS, in an environment of diminished antioxidant activity. The dopamine-oxidising enzyme MAO-B, which resides in the outer membrane of mitochondria, can also be inhibited by some chelators.
References
- de Lau LM, Breteler MM. Epidemiology of Parkinson’s disease. The Lancet Neurology. 2006;5(6):525–535. - PubMed
- Dauer W, Przedborski S. Parkinson’s disease: mechanisms and models. Neuron. 2003;39(6):889–909. - PubMed
- Rausch W, Hirata Y, Nagatsu T, Riederer P, Jellinger K. Tyrosine hydroxylase activity in caudate nucleus from Parkinson’s disease: effects of iron and phosphorylating agents. Journal of Neurochemistry. 1988;50(1):202–208. - PubMed
- Dexter DT, Wells FR, Agid F, et al. Increased nigral iron content in postmortem Parkinsonian brain. Lancet. 1987;2(8569):1219–1220. - PubMed
- Sofic E, Riederer P, Heinsen H, et al. Increased iron (III) and total iron content in post mortem substantia nigra of Parkinsonian brain. Journal of Neural Transmission, Supplementa. 1988;74(3):199–205. - PubMed
LinkOut - more resources
Full Text Sources