Protective role of Toll-like Receptor 3-induced type I interferon in murine coronavirus infection of macrophages - PubMed (original) (raw)
Protective role of Toll-like Receptor 3-induced type I interferon in murine coronavirus infection of macrophages
Liudmila Mazaleuskaya et al. Viruses. 2012 May.
Abstract
Toll-like Receptors (TLRs) sense viral infections and induce production of type I interferons (IFNs), other cytokines, and chemokines. Viral recognition by TLRs and other pattern recognition receptors (PRRs) has been proven to be cell-type specific. Triggering of TLRs with selected ligands can be beneficial against some viral infections. Macrophages are antigen-presenting cells that express TLRs and have a key role in the innate and adaptive immunity against viruses. Coronaviruses (CoVs) are single-stranded, positive-sense RNA viruses that cause acute and chronic infections and can productively infect macrophages. Investigation of the interplay between CoVs and PRRs is in its infancy. We assessed the effect of triggering TLR2, TLR3, TLR4, and TLR7 with selected ligands on the susceptibility of the J774A.1 macrophage cell line to infection with murine coronavirus (mouse hepatitis virus, [MHV]). Stimulation of TLR2, TLR4, or TLR7 did not affect MHV production. In contrast, pre-stimulation of TLR3 with polyinosinic-polycytidylic acid (poly I:C) hindered MHV infection through induction of IFN-β in macrophages. We demonstrate that activation of TLR3 with the synthetic ligand poly I:C mediates antiviral immunity that diminishes (MHV-A59) or suppresses (MHV-JHM, MHV-3) virus production in macrophages.
Keywords: TLR; antiviral state; macrophage; murine coronavirus; poly I:C; type I IFN.
Figures
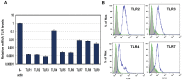
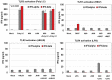
Figure 1
Expression of Toll-like Receptors (TLRs) in J774A.1 murine macrophages. (A) J774A.1 cells were profiled for TLR1-9 gene expression by quantitative real-time polymerase chain reaction (PCR) using predeveloped TaqMan Gene Expression assays (AppliedBiosystems). Expression levels of target genes were normalized to the housekeeping gene β-actin (ΔCt). Relative gene expression values were calculated based on the ΔΔCt method, with data for all samples analyzed against the mean value of four replicates; (B) Expression of cell surface TLR2 and TLR4, and intracellular TLR3 and TLR7 in naïve J774A.1 cells was analyzed by flow cytometry (FACS) using standard protocols. Empty, dashed and blue histograms represent only cells (no antibodies), isotype antibody controls, and TLR expression in 774A.1 cells, respectively.
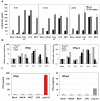
Figure 2
Induction of proinflammatory cytokine response and type I IFN after triggering with ligands specific to TLR2-TLR4, and TLR7 in J774A.1 cells. (A) J774A.1 macrophages were stimulated with 108 cells/mL HKLM (TLR2), 1 μg/mL poly I:C (TLR3), 5 μg/mL LPS (TLR4), or 5 μg/mL imiquimod (R837) (TLR7) for 6, 18 and 24 h. Cell-free supernatants were assessed for the production of IL-6 and TNF-α using the enzyme-linked immunosorbent assay (ELISA). Error bars represent the standard error of the mean of two replicates; (B) Real-Time PCR of type I IFN gene expression in TLR stimulated J774A.1 macrophages. J774A.1 was profiled for IFNβ and IFNα4 gene expression by quantitative real-time PCR using predeveloped TaqMan Gene Expression assays (AppliedBiosystems). Expression levels of target genes were normalized to the housekeeping gene 18S rRNA (ΔCt). Gene expression values were calculated based on the ΔΔCt method, with data for all samples analyzed against the mean value of four replicates. Error bars represent the standard error of the mean of two independent experiments, each done in duplicate; (C) Type I IFN production in TLR2-4 and TLR7 activated J774A.1 cells. J774A.1 macrophages were stimulated with 108 cells/mL HKLM (TLR2), 0.25 μg/mL poly I:C (TLR3), 5 μg/mL LPS (TLR4), or 5 μg/mL imiquimod (R837) (TLR7) for 6 h. Supernatants collected 6 h after TLR stimulation were assessed for IFN-α and IFN-β production using ELISA. Error bars represent the standard error of the mean of two independent experiments, each done in duplicate.
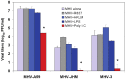
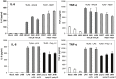
Figure 3
Effect of triggering TLR2, TLR3, TLR4, and TLR7 on virus production in MHV-infected J774A.1 macrophages. J774A.1 cells were prestimulated with TLR ligands for 6 h, infected with MHV-A59, MHV-JHM or MHV-3 (1 MOI) by adsorption for 1h in the absence of the ligands, and activated for up to 18 h p.i. with the appropriate TLR agonist. TLR ligands were used as follows: HKLM (TLR2) at 108 cells/mL; LPS (TLR4) at 5 μg/mL; Imiquimod (R837) (TLR7) at 5 μg/mL. Poly I:C (TLR3) was tested at a range of concentrations (0.25, 0.5, and 1.0 μg/mL). Because poly I:C triggered a comparable effect on MHV production at all concentrations (data not shown), viral titers at 0.25 μg/mL were included in the plot. Cells incubated with the basal medium before and during infection served as a negative control for the effect of TLR triggering on virus production. MHV titers were assessed in cell-free supernatants using a plaque assay on L2 fibroblasts. The data shown are the mean viral titers of three independent experiments, each done in duplicate ± standard deviation (*p value relative to virus alone, p < 0.001 Student’s t test).
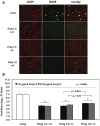
Figure 4
Prestimulation with poly I:C before virus adsorption is sufficient to trigger a profound antiviral effect in MHV-infected macrophages. (A) J774A.1 macrophages were prestimulated with poly I:C for 6 h and coactivated during RA59-GFP (1 MOI) infection for 18 h postadsorption at concentrations of 0.25 and 1.0 μg/mL of the TLR3 ligand. Cells were treated as follows: (1) poly I:C prestimulated only (poly I:C +/−); (2) poly I:C coactivated only after virus adsorption (poly I:C −/+); and (3) poly I:C-treated before and after virus adsorption (poly I:C +/+). Unstimulated but infected macrophages served as a negative control for poly I:C antiviral effect. RA59-GFP infection was visualized at the original magnification x100. The data shown are representative images of two independent wells for cells treated with 0.25 μg/mL poly I:C. Original magnification x100. (B) RA59-GFP titers were assessed in cell-free supernatants from (A) using a plaque assay on L2 fibroblasts. Error bars represent the standard error of the mean of two replicates (* p value relative to virus alone; other p values relative to Poly I:C-pre-stimulated cells only, Student’s t test).
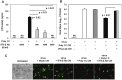
Figure 5
Involvement of soluble factors in the antiviral effect mediated by TLR3 in poly I:C-stimulated, MHV-infected macrophages. J774A.1 macrophages were pretreated for 3 h with the conditioned medium (CM) from macrophages prestimulated with TLR ligands for 6 h from Figure 2. CM was diluted 1:1 with the basal medium to replenish nutrients. Then cells were infected with RA59-GFP (1 MOI) for 1 h adsorption and incubated in the basal medium for up to 18 h p.i. Cells pretreated with the CM from mock cells or with fresh basal medium served as negative controls for the TLR-triggered effect. RA59-GFP titers were assessed in cell-free supernatants using a plaque assay on L2 fibroblasts. Error bars represent the standard error of the mean of two replicates (p value relative to cells pretreated with CM from mock, p < 0.01, Student’s t test).
Figure 6
Type I IFN production in cell-free supernatants from J774A.1 macrophages stimulated with TLR ligands, MHV-infected, and/or coactivated during MHV infection. J774A.1 cells were prestimulated for 6 h with HKLM (108 cells/mL), LPS (5 μg/mL), R837 (5 μg/mL), and poly I:C (0.25 μg/mL); media was removed and: (1) a second challenge of the corresponding TLR ligand (same concentrations) was added to the cells for 18 h; (2) cells were prestimulated for 6 h with the TLR ligands as above; media was removed and cells infected with MHV-A59 or MHV-JHM at 1.0 MOI for 1 h of adsorption in the absence of the ligands; after virus adsorption cells were stimulated with a second challenge of the TLR ligands using the same concentrations as during prestimulation and samples were taken at 18 h; (3) cells were not TLR activated and only infected with MHV-A59 or MHV-JHM at 1.0 MOI for 1 h of adsorption in the absence of the ligands; fresh media was added to the cells for 18 h. Non-stimulated, non-infected J774A.1 cells were used as mock control. Cell-free supernatants were taken at 18 h from TLR-activated alone, infected alone, and TLR-activated and infected and assessed for IFN-α and IFN-β production using ELISA. Error bars represent the standard error of the mean of two independent experiments, each done in duplicate; * p < 0.05, Student’s t test.
Figure 7
IL-6 and TNF-α production in cell-free supernatants from J774A.1 macrophages stimulated with TLR ligands, MHV-infected, and/or coactivated during MHV infection. J774A.1 cells were prestimulated for 6 h with HKLM (108 cells/mL), LPS (5 μg/mL), R837 (5 μg/mL), and poly I:C (0.25 μg/mL); media was removed and: 1) a second challenge of the corresponding TLR ligand (same concentrations) was added to the cells for 18 h; 2) cells were prestimulated for 6 h with the TLR ligands as above; media was removed and cells infected with MHV-A59 or MHV-JHM at 1.0 MOI for 1 h of adsorption in the absence of the ligands; after virus adsorption cells were stimulated with a second challenge of the TLR ligands using the same concentrations as during prestimulation and samples were taken at 18 h; 3) cells were not TLR activated and only infected with MHV-A59 or MHV-JHM at 1.0 MOI for 1 h of adsorption in the absence of the ligands; fresh media was added to the cells for 18 h. Non-stimulated, non-infected J774A.1 cells were used as mock control. Cell-free supernatants were taken at 18 h from TLR-activated alone, infected alone, and TLR-activated and infected and assessed for IL-6 and TNF-α using ELISA. Error bars represent the standard error of the mean of two independent experiments, each done in duplicate.
Figure 8
Role of IFN-β in poly I:C-triggered anti-viral response in MHV-infected macrophages. (A) Anti-IFN-β Ab was titrated in the presence of poly I:C in J774A.1 macrophages. Cells were activated with 0.25 μg/mL poly I:C with or without anti-IFN-β Abs at 500, 1000, 2000 U/mL. Supernatants were collected after 6 h, cleared of cell debris and assessed with IFN-β ELISA. Error bars represent the standard error of the mean of two replicates (p value relative to cells activated with poly I:C alone, Student’s t test). ND, not detected; (B) RA59-GFP titers were assessed in cell-free supernatants from (C) using a plaque assay on L2 fibroblasts. Error bars represent the standard error of the mean of two replicates (p value relative to cells pre-treated with Poly I:C conditioned medium, Student’s t test); (C) J774A.1 macrophages were pretreated for 3 h with the conditioned medium (CM) from the anti-IFN-β Ab titration assay in (A). CM was diluted 1:1 with the basal medium to replenish nutrients. Then cells were infected with RA59-GFP (1 MOI) for 1 h adsorption and incubated in the basal medium for up to 18h p.i. Cells pretreated with the CM from mock cells or fresh basal medium (untreated) served as negative controls for the poly I:C+/-anti-IFN-β Ab-triggered effect. RA59-GFP infection was visualized at the original magnification x100. The data shown are representative images of two independent wells per treatment (p values, Student’s t test).
Similar articles
- A role for microRNA-155 modulation in the anti-HIV-1 effects of Toll-like receptor 3 stimulation in macrophages.
Swaminathan G, Rossi F, Sierra LJ, Gupta A, Navas-Martín S, Martín-García J. Swaminathan G, et al. PLoS Pathog. 2012 Sep;8(9):e1002937. doi: 10.1371/journal.ppat.1002937. Epub 2012 Sep 20. PLoS Pathog. 2012. PMID: 23028330 Free PMC article. - Murine coronavirus mouse hepatitis virus is recognized by MDA5 and induces type I interferon in brain macrophages/microglia.
Roth-Cross JK, Bender SJ, Weiss SR. Roth-Cross JK, et al. J Virol. 2008 Oct;82(20):9829-38. doi: 10.1128/JVI.01199-08. Epub 2008 Jul 30. J Virol. 2008. PMID: 18667505 Free PMC article. - Selective Packaging in Murine Coronavirus Promotes Virulence by Limiting Type I Interferon Responses.
Athmer J, Fehr AR, Grunewald ME, Qu W, Wheeler DL, Graepel KW, Channappanavar R, Sekine A, Aldabeeb DS, Gale M Jr, Denison MR, Perlman S. Athmer J, et al. mBio. 2018 May 1;9(3):e00272-18. doi: 10.1128/mBio.00272-18. mBio. 2018. PMID: 29717007 Free PMC article. - Antiviral signaling through pattern recognition receptors.
Kawai T, Akira S. Kawai T, et al. J Biochem. 2007 Feb;141(2):137-45. doi: 10.1093/jb/mvm032. Epub 2006 Dec 26. J Biochem. 2007. PMID: 17190786 Review. - Human Toll-like receptor-dependent induction of interferons in protective immunity to viruses.
Zhang SY, Jouanguy E, Sancho-Shimizu V, von Bernuth H, Yang K, Abel L, Picard C, Puel A, Casanova JL. Zhang SY, et al. Immunol Rev. 2007 Dec;220(1):225-36. doi: 10.1111/j.1600-065X.2007.00564.x. Immunol Rev. 2007. PMID: 17979850 Free PMC article. Review.
Cited by
- Engineering a multi epitope vaccine against SARS-CoV-2 by exploiting its non structural and structural proteins.
Rajput VS, Sharma R, Kumari A, Vyas N, Prajapati V, Grover A. Rajput VS, et al. J Biomol Struct Dyn. 2022;40(19):9096-9113. doi: 10.1080/07391102.2021.1924265. Epub 2021 May 26. J Biomol Struct Dyn. 2022. PMID: 34038700 Free PMC article. - Immunoediting in SARS-CoV-2: Mutual relationship between the virus and the host.
Kheshtchin N, Bakhshi P, Arab S, Nourizadeh M. Kheshtchin N, et al. Int Immunopharmacol. 2022 Apr;105:108531. doi: 10.1016/j.intimp.2022.108531. Epub 2022 Jan 10. Int Immunopharmacol. 2022. PMID: 35074569 Free PMC article. Review. - Long COVID Definition, Symptoms, Risk Factors, Epidemiology and Autoimmunity: A Narrative Review.
Kozłowski P, Leszczyńska A, Ciepiela O. Kozłowski P, et al. Am J Med Open. 2024 Feb 14;11:100068. doi: 10.1016/j.ajmo.2024.100068. eCollection 2024 Jun. Am J Med Open. 2024. PMID: 39034937 Free PMC article. - Inhibition of dengue and chikungunya virus infections by RIG-I-mediated type I interferon-independent stimulation of the innate antiviral response.
Olagnier D, Scholte FE, Chiang C, Albulescu IC, Nichols C, He Z, Lin R, Snijder EJ, van Hemert MJ, Hiscott J. Olagnier D, et al. J Virol. 2014 Apr;88(8):4180-94. doi: 10.1128/JVI.03114-13. Epub 2014 Jan 29. J Virol. 2014. PMID: 24478443 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
- DK089314/DK/NIDDK NIH HHS/United States
- R21 AI088423/AI/NIAID NIH HHS/United States
- R21 DK089314/DK/NIDDK NIH HHS/United States
- AI088423/AI/NIAID NIH HHS/United States
- R03 NS061179/NS/NINDS NIH HHS/United States
- R01 NS065727/NS/NINDS NIH HHS/United States
- NS061179/NS/NINDS NIH HHS/United States
- NS065727/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources