Human intestinal lumen and mucosa-associated microbiota in patients with colorectal cancer - PubMed (original) (raw)
Human intestinal lumen and mucosa-associated microbiota in patients with colorectal cancer
Weiguang Chen et al. PLoS One. 2012.
Abstract
Recent reports have suggested the involvement of gut microbiota in the progression of colorectal cancer (CRC). We utilized pyrosequencing based analysis of 16S rRNA genes to determine the overall structure of microbiota in patients with colorectal cancer and healthy controls; we investigated microbiota of the intestinal lumen, the cancerous tissue and matched noncancerous normal tissue. Moreover, we investigated the mucosa-adherent microbial composition using rectal swab samples because the structure of the tissue-adherent bacterial community is potentially altered following bowel cleansing. Our findings indicated that the microbial structure of the intestinal lumen and cancerous tissue differed significantly. Phylotypes that enhance energy harvest from diets or perform metabolic exchange with the host were more abundant in the lumen. There were more abundant Firmicutes and less abundant Bacteroidetes and Proteobacteria in lumen. The overall microbial structures of cancerous tissue and noncancerous tissue were similar; however the tumor microbiota exhibited lower diversity. The structures of the intestinal lumen microbiota and mucosa-adherent microbiota were different in CRC patients compared to matched microbiota in healthy individuals. Lactobacillales was enriched in cancerous tissue, whereas Faecalibacterium was reduced. In the mucosa-adherent microbiota, Bifidobacterium, Faecalibacterium, and Blautia were reduced in CRC patients, whereas Fusobacterium, Porphyromonas, Peptostreptococcus, and Mogibacterium were enriched. In the lumen, predominant phylotypes related to metabolic disorders or metabolic exchange with the host, Erysipelotrichaceae, Prevotellaceae, and Coriobacteriaceae were increased in cancer patients. Coupled with previous reports, these results suggest that the intestinal microbiota is associated with CRC risk and that intestinal lumen microflora potentially influence CRC risk via cometabolism or metabolic exchange with the host. However, mucosa-associated microbiota potentially affects CRC risk primarily through direct interaction with the host.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
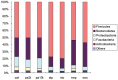
Figure 1. Relative abundance of bacterial phyla in microbiota of seven groups of samples.
“Others” represents the unclassified bacteria, Chloroflexi, Deferribacteres, Chlorobi, Deinococcus-Thermus, Acidobacteria, Planctomycetes, Lentisphaerae, Spirochaetes, Synergistetes, Tenericutes, Verrucomicrobia and Cyanobacteria. The first eight phyla were not apparent in stool samples, and the first four phyla were not apparent in swab samples.
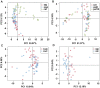
Figure 2. PCA plots based on unweighted Unifrac metrics.
Each symbol represents a sample. (A) group cat, stp and swp; (B) group cat, pa2t and pa10t; (C) group swp and swc; (D) group stp and stc.
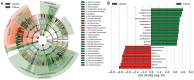
Figure 3. Different structures of intestinal lumen and cancerous tissue microbiota.
(A) Taxonomic representation of statistically and biologically consistent differences between cancerous tissue and intestinal lumen. Differences are represented by the color of the most abundant class (Red indicating cancerous tissue, yellow non-significant and green intestinal lumen). The diameter of each circle’s diameter is proportional to the taxon’s abundance. (B) Histogram of the LDA scores for differentially abundant genera. Cladogram was calculated by LEfSe, a metagenome analysis approach which performs the linear discriminant analysis following the Wilcoxon Mann-Whitney test to assess effect size of each differentially abundant taxon or OTU; the cladogram is displayed according to effect size.
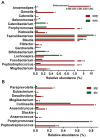
Figure 4. Relative abundance of significantly different genera between CRC patients and healthy controls.
(A) Genera different between swp and swc. (B) Genera differing between stp and stc. The Mann-Whitney test was used to evaluate the importance of comparisons between indicated groups. *P<0.05, **P<0.01.
References
- Ley RE, Peterson DA, Gordon JI. Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell. 2006;124:837–848. - PubMed
- Rowland IR. The role of the gastrointestinal microbiota in colorectal cancer. Curr Pharm Des. 2009;15:1524–1527. - PubMed
- Candela M, Guidotti M, Fabbri A, Brigidi P, Franceschi C, et al. Human intestinal microbiota: cross-talk with the host and its potential role in colorectal cancer. Crit Rev Microbiol. 2011;37:1–14. - PubMed
- Wei H, Dong L, Wang T, Zhang M, Hua W, et al. Structural shifts of gut microbiota as surrogate endpoints for monitoring host health changes induced by carcinogen exposure. FEMS Microbiol Ecol. 2010;73:577–586. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical