Perinuclear mitochondrial clustering creates an oxidant-rich nuclear domain required for hypoxia-induced transcription - PubMed (original) (raw)
Perinuclear mitochondrial clustering creates an oxidant-rich nuclear domain required for hypoxia-induced transcription
Abu-Bakr Al-Mehdi et al. Sci Signal. 2012.
Abstract
Mitochondria can govern local concentrations of second messengers, such as reactive oxygen species (ROS), and mitochondrial translocation to discrete subcellular regions may contribute to this signaling function. Here, we report that exposure of pulmonary artery endothelial cells to hypoxia triggered a retrograde mitochondrial movement that required microtubules and the microtubule motor protein dynein and resulted in the perinuclear clustering of mitochondria. This subcellular redistribution of mitochondria was accompanied by the accumulation of ROS in the nucleus, which was attenuated by suppressing perinuclear clustering of mitochondria with nocodazole to destabilize microtubules or with small interfering RNA-mediated knockdown of dynein. Although suppression of perinuclear mitochondrial clustering did not affect the hypoxia-induced increase in the nuclear abundance of hypoxia-inducible factor 1α (HIF-1α) or the binding of HIF-1α to an oligonucleotide corresponding to a hypoxia response element (HRE), it eliminated oxidative modifications of the VEGF (vascular endothelial growth factor) promoter. Furthermore, suppression of perinuclear mitochondrial clustering reduced HIF-1α binding to the VEGF promoter and decreased VEGF mRNA accumulation. These findings support a model for hypoxia-induced transcriptional regulation in which perinuclear mitochondrial clustering results in ROS accumulation in the nucleus and causes oxidative base modifications in the VEGF HRE that are important for transcriptional complex assembly and VEGF mRNA expression.
Conflict of interest statement
Competing interests: The authors declare that they have no competing interests.
Figures
Fig. 1
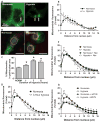
Perinuclear mitochondrial clustering in hypoxia: role of microtubules and the dynein motor system. (A) Left image: The arrow points to the nucleus of a capillary endothelial cell in a perfused rat lung. Mitochondrial labeling is green. Right image: Arrows point to clustered mitochondria after 3 hours of hypoxia. Scale bar, 20 μm. (B) Rat PAECs stained with MitoTracker Red, Oregon Green paclitaxel (microtubules), and Hoechst 33342 (nuclei, blue) were cultured under normoxia or hypoxia for 3 hours. Yellow circle denotes perinuclear region. Scale bar, 30 μm. (C) Quantification of perinuclear mitochondrial distribution in normoxic (NORM) and hypoxic PAECs. (D) Distribution of mitochondria in concentric rings radiating from the nucleus outward in normoxic and hypoxic PAECs. (E) Mitochondrial distribution in normoxic and hypoxic PASMCs. (F) Impact of the microtubule-destabilizing agent nocodazole (Noc) on mitochondrial distribution in normoxic and hypoxic PAECs. (G) Impact of siRNA knockdown of the dynein heavy chain (siDYN) on mitochondrial distribution in normoxic and hypoxic PAECs. n = 6 different culture dishes with three to six cells analyzed per dish. *P < 0.05, different from normoxia.
Fig. 2
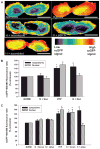
Impact of perinuclear mitochondrial clustering on hypoxia-induced pan-cellular ROS generation. (A) Pseudo-colored intensity plots of roGFP signal in normoxic PAECs and PAECs cultured in hypoxia (H) or cultured in hypoxia in the presence of nocodazole or after siRNA-mediated dynein knockdown (siDYN). roGFP signal intensity (indicated by the color bar) correlates with ROS concentrations. Scale bar, 15 μm. (B) Quantitative assessment of nuclear and cytoplasmic roGFP signals in the absence and presence of nocodazole (Noc) in normoxic (N) and hypoxic (H) PAECs. (C) Effect of dynein heavy chain–specific siRNA (siDYN) or scrambled siRNA (Scram) on hypoxia-induced changes in nuclear and cytoplasmic roGFP signals. n = 3 to 5 different culture dishes with three to six cells analyzed per dish for all panels. *P < 0.05, increased from normoxia.
Fig. 3
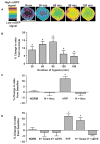
Hypoxia induces a redistribution of nuclear ROS that requires microtubule- and dynein-dependent perinuclear mitochondrial clustering. (A) Time-dependent effects of hypoxia on nuclear-targeted roGFP signals as depicted by pseudo-colored, ratiometric images of a PAEC nucleus. roGFP signal intensity (indicated by the color bar) correlates with ROS concentrations. Scale bar, 15 μm. (B) Time-dependent effects of hypoxia on nuclear roGFP fluorescence ratio in PAECs. (C) Effect of nocodazole on hypoxia-induced changes in roGFP fluorescence ratio in normoxic (NORM, N) PAECs and in PAECs cultured in hypoxia (HYP, H) for 60 min. (D) Effect of dynein-specific siRNA (siDYN) or scrambled siRNA (Scram) on roGFP fluorescence ratio in normoxic and hypoxic PAECs. n = 3 to 5 different culture dishes with 3 to 6 cells analyzed per dish for all panels. *P < 0.05, increased from normoxia.
Fig. 4
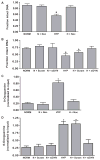
Hypoxia causes oxidative base modifications in the HRE of the VEGF promoter that require perinuclear mitochondrial clustering. (A) Effect of nocodazole on Fpg-detectable oxidative base damage in the VEGF HRE in PAECS cultured in normoxia (NORM, N) or hypoxia (HYP, H). (B) Effect of dynein-specific siRNA (siDYN) or scrambled siRNA (Scram) on Fpg-detectable oxidative base damage in the VEGF HRE in normoxic and hypoxic PAECS. (C) ChIP analysis of 8-oxoguanine–containing VEGF HRE sequences in PAECs treated with nocodazole. (D) ChIP analysis of 8-oxoguanine–containing HRE sequences in PAECs transfected with dynein-specific siRNA or scrambled siRNA. n = 4 to 6 different culture dishes for all panels. *P < 0.05, increased from normoxia.
Fig. 5
Base modifications in the VEGF HRE that occur after perinuclear clustering mitochondria are required for HIF-1α binding and VEGF mRNA expression. (A) Top: Western analyses of HIF-1α and the nuclear marker lamin A/C in PAECs cultured for 3 hours under normoxia (NORM) or hypoxia (HYP) in the presence of nocodazole (Noc) or after transfection with dynein-specific siRNA (siDYN). Representative of four experiments. Bottom: Quantification of HIF-1α abundance normalized to lamin A/C calculated as a percentage of the normoxic control. n = 4 separate culture dishes per experimental group. *P < 0.05, increased from normoxia. (B) Western blot analysis of HIF-1α associating with a 65-mer oligonucleotide model of the VEGF HRE (DNA affinity precipitation analysis). Oligonucleotide-associated HIF-1α was derived from nuclear extracts isolated from normoxic and hypoxic control PAECs or PAECs treated with nocodazole or transfected with dynein-specific siRNA. Data are representative of three separate experiments. (C) ChIP analysis of VEGF HRE sequences immunoprecipitating with HIF-1α recovered from PAECs incubated under normoxia or hypoxia in the presence of nocodazole. (D) ChIP assays for VEGF HRE sequences immunoprecipitating with HIF-1α from PAECs transfected with dynein-specific (siDYN) or scrambled siRNA (Scram). (E) Quantitative RT-PCR analysis of VEGF mRNA expression by PAECs in the presence of nocodazole. (F) Quantitative RT-PCR analysis of VEGF mRNA expression in PAECs transfected with dynein-specific or scrambled siRNA. n = 4 to 6 separate culture dishes per experimental group. *P < 0.05, increased from normoxia. **P < 0.05, different from normoxia and hypoxia alone.
Similar articles
- An oxidative DNA "damage" and repair mechanism localized in the VEGF promoter is important for hypoxia-induced VEGF mRNA expression.
Pastukh V, Roberts JT, Clark DW, Bardwell GC, Patel M, Al-Mehdi AB, Borchert GM, Gillespie MN. Pastukh V, et al. Am J Physiol Lung Cell Mol Physiol. 2015 Dec 1;309(11):L1367-75. doi: 10.1152/ajplung.00236.2015. Epub 2015 Oct 2. Am J Physiol Lung Cell Mol Physiol. 2015. PMID: 26432868 Free PMC article. - HIF-1α protects against oxidative stress by directly targeting mitochondria.
Li HS, Zhou YN, Li L, Li SF, Long D, Chen XL, Zhang JB, Feng L, Li YP. Li HS, et al. Redox Biol. 2019 Jul;25:101109. doi: 10.1016/j.redox.2019.101109. Epub 2019 Jan 14. Redox Biol. 2019. PMID: 30686776 Free PMC article. - A potential role for reactive oxygen species and the HIF-1alpha-VEGF pathway in hypoxia-induced pulmonary vascular leak.
Irwin DC, McCord JM, Nozik-Grayck E, Beckly G, Foreman B, Sullivan T, White M, T Crossno J Jr, Bailey D, Flores SC, Majka S, Klemm D, van Patot MC. Irwin DC, et al. Free Radic Biol Med. 2009 Jul 1;47(1):55-61. doi: 10.1016/j.freeradbiomed.2009.03.027. Epub 2009 Apr 7. Free Radic Biol Med. 2009. PMID: 19358884 Free PMC article. - Modulating mitochondrial intracellular location as a redox signal.
Murphy MP. Murphy MP. Sci Signal. 2012 Sep 18;5(242):pe39. doi: 10.1126/scisignal.2003386. Sci Signal. 2012. PMID: 22990116 Review. - Mitochondrial metabolism, redox signaling, and fusion: a mitochondria-ROS-HIF-1alpha-Kv1.5 O2-sensing pathway at the intersection of pulmonary hypertension and cancer.
Archer SL, Gomberg-Maitland M, Maitland ML, Rich S, Garcia JG, Weir EK. Archer SL, et al. Am J Physiol Heart Circ Physiol. 2008 Feb;294(2):H570-8. doi: 10.1152/ajpheart.01324.2007. Epub 2007 Dec 14. Am J Physiol Heart Circ Physiol. 2008. PMID: 18083891 Review.
Cited by
- Chloroplast Stromules Function during Innate Immunity.
Caplan JL, Kumar AS, Park E, Padmanabhan MS, Hoban K, Modla S, Czymmek K, Dinesh-Kumar SP. Caplan JL, et al. Dev Cell. 2015 Jul 6;34(1):45-57. doi: 10.1016/j.devcel.2015.05.011. Epub 2015 Jun 25. Dev Cell. 2015. PMID: 26120031 Free PMC article. - The Influence of Reactive Oxygen Species in the Immune System and Pathogenesis of Multiple Sclerosis.
Tavassolifar MJ, Vodjgani M, Salehi Z, Izad M. Tavassolifar MJ, et al. Autoimmune Dis. 2020 Jun 25;2020:5793817. doi: 10.1155/2020/5793817. eCollection 2020. Autoimmune Dis. 2020. PMID: 32789026 Free PMC article. Review. - Deep Analysis of Mitochondria and Cell Health Using Machine Learning.
Zahedi A, On V, Phandthong R, Chaili A, Remark G, Bhanu B, Talbot P. Zahedi A, et al. Sci Rep. 2018 Nov 5;8(1):16354. doi: 10.1038/s41598-018-34455-y. Sci Rep. 2018. PMID: 30397207 Free PMC article. - Development and application of a UHPLC-MS/MS metabolomics based comprehensive systemic and tissue-specific screening method for inflammatory, oxidative and nitrosative stress.
Schoeman JC, Harms AC, van Weeghel M, Berger R, Vreeken RJ, Hankemeier T. Schoeman JC, et al. Anal Bioanal Chem. 2018 Apr;410(10):2551-2568. doi: 10.1007/s00216-018-0912-2. Epub 2018 Mar 2. Anal Bioanal Chem. 2018. PMID: 29497765 Free PMC article. - Functional Consequences of PDK4 Deficiency in Doberman Pinscher Fibroblasts.
Bolfer L, Estrada AH, Larkin C, Conlon TJ, Lourenco F, Taggart K, Suzuki-Hatano S, Pacak CA. Bolfer L, et al. Sci Rep. 2020 Mar 3;10(1):3930. doi: 10.1038/s41598-020-60879-6. Sci Rep. 2020. PMID: 32127618 Free PMC article.
References
- Bell EL, Chandel NS. Mitochondrial oxygen sensing: Regulation of hypoxia-inducible factor by mitochondrial generated reactive oxygen species. Essays Biochem. 2007;43:17–27. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 8R01OD010944-03/OD/NIH HHS/United States
- R01HL113614/HL/NHLBI NIH HHS/United States
- P01HL66299/HL/NHLBI NIH HHS/United States
- R01 HL113614/HL/NHLBI NIH HHS/United States
- P01 HL066299/HL/NHLBI NIH HHS/United States
- R01 HL073244/HL/NHLBI NIH HHS/United States
- R01HL58234/HL/NHLBI NIH HHS/United States
- R01 OD010944/OD/NIH HHS/United States
- R01 HL058234/HL/NHLBI NIH HHS/United States
- R01 RR031286/RR/NCRR NIH HHS/United States
- R01HL073244/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources