miR-33 controls the expression of biliary transporters, and mediates statin- and diet-induced hepatotoxicity - PubMed (original) (raw)
miR-33 controls the expression of biliary transporters, and mediates statin- and diet-induced hepatotoxicity
Ryan M Allen et al. EMBO Mol Med. 2012 Sep.
Abstract
Bile secretion is essential for whole body sterol homeostasis. Loss-of-function mutations in specific canalicular transporters in the hepatocyte disrupt bile flow and result in cholestasis. We show that two of these transporters, ABCB11 and ATP8B1, are functional targets of miR-33, a micro-RNA that is expressed from within an intron of SREBP-2. Consequently, manipulation of miR-33 levels in vivo with adenovirus or with antisense oligonucleotides results in changes in bile secretion and bile recovery from the gallbladder. Using radiolabelled cholesterol, we show that systemic silencing of miR-33 leads to increased sterols in bile and enhanced reverse cholesterol transport in vivo. Finally, we report that simvastatin causes, in a dose-dependent manner, profound hepatotoxicity and lethality in mice fed a lithogenic diet. These latter results are reminiscent of the recurrent cholestasis found in some patients prescribed statins. Importantly, pretreatment of mice with anti-miR-33 oligonucleotides rescues the hepatotoxic phenotype. Therefore, we conclude that miR-33 mediates some of the undesired, hepatotoxic effects of statins.
Copyright © 2012 EMBO Molecular Medicine.
Figures
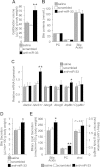
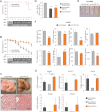
Figure 1. Increased bile secretion following silencing of miR-33
- Bile recovered from the gallbladder of mice (n = 5) on chow diet, injected with saline, and scrambled or anti-miR-33 oligonucleotides (5 mpk i.v., for 2 consecutive days). Animals were then kept for a week on chow and fasted overnight before sample collection.
- Levels of phosphatidylcholine (PC), cholesterol (chol) and bile acids present in gallbladder bile in the same mice.
- Relative expression of hepatic canalicular transporters in the same mice.
- A different group of mice (n = 6–8) was injected as described above. A week later, mice were anesthetized, the bile duct cannulated, and hepatic bile collected for 1 h.
- Levels of bile acids, phosphatidylcholine (PC), cholesterol (chol) present in bile from the last group of mice. Data shown as mean ± SEM. *p < 0.05; **p < 0.01.
Figure 2. Functional miR-33 responsive elements in the 3′UTR of ATP8B1 and ABCB11
- A,B. Conserved sequences in the 3′UTR of ATP8B1 and ABCB11 are partially complementary to miR-33. The element in human ATP8B1 is located 1877–1897 nt after the stop codon. The element in ABCB11 overlaps the stop codon in primates, while rodents show a conserved sequence 732–751 nt after the stop codon.
- C,D. Luciferase assays in HEK293 cells using the whole 3′UTR of human or murine ATP8B1 and ABCB11, or a single copy of the responsive elements (RE) identified above, or mutant responsive elements (RE*; where AATGCA was mutated to GGGTTG to prevent complementarity to the seed sequence of the miRNA), co-transfected with (closed bars) or without (open bars) a vector to overexpress miR-33. In grey, data from empty (negative control) and R33 (positive control containing a 100% match to miR-33) reporter vectors.
- E,F. Relative mRNA expression of canalicular transporters in primary murine hepatocytes (n = 4 dishes/condition) and human HuH-7 hepatoma cells (n = 3 dishes/condition) transduced 48 h with empty or miR-33 adenovirus.
- G. Relative protein levels in HuH7 cells transduced with empty or miR-33 adenovirus. Some cells were incubated for 16 h in the presence of FXR:RXR agonists (2 µmol/L GW4064 : 1 µmol/L 9-_cis_-retinoic acid) to induce ABCB11. Asterisk indicates a non-specific band. Data shown as mean ± SD; **p < 0.01.
Figure 3. Effect of miR-33 overexpression on diet-induced cholestasis
- C57BL/6 mice (n = 5–7) were kept on chow diet or lithogenic diet for 7 days (lanes 1–2). A different group of animals were transduced i.v. with empty or miR-33 adenovirus (2 × 109 pfu/mouse), and then switched to the lithogenic diet (lanes 3–4). After 7 days, mice were fasted overnight and killed the following morning. Data show relative levels of hepatic miR-33.
- The volume of bile recovered from the gallbladder is significantly reduced in mice transduced with miR-33. Picture shows pooled bile collected from five mice each in an independent experiment.
- Liver to body mass ratios.
- Hepatic levels of bile acids; total, unesterified (UC) and esterified (EC) cholesterol; phosphatidylcholine (PC) and triglycerides (TG).
- The amounts of bile acids (BA), cholesterol (chol) and phosphatidylcholine (PC) were determined in bile, and expressed as mol% (mol per 100 mol). Compared to mice infused with saline or Adeno-empty, the bile from animals transduced with Adeno-miR-33 showed increased amounts of cholesterol (11.6 ± 1.1 vs. 6.6 ± 0.5 vs. 5.9 ± 0.6 mol%; miR _v_s. scrambled vs. saline; p = 0.009), and decreased amounts of bile acids (78.6 ± 1.7 vs. 83.7 ± 1.0 vs. 82.1 ± 1.4 mol%; miR _v_s. scrambled vs. saline; p = 0.03), but no change in PC contents (9.8 ± 1.3 vs. 9.7 ± 0.6 vs. 12.0 ± 0.9 mol%; miR vs. scrambled vs. saline).
- Relative expression of hepatic canalicular transporters in the same mice. Data shown as mean ± SD; *p < 0.05; **p < 0.01.
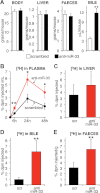
Figure 4. Reverse cholesterol transport is enhanced after systemic miR-33 silencing
C57BL/6 mice (n = 7) were infused i.v. with 5 mpk scrambled or anti-miR-33 oligonucleotides for 2 consecutive days, and 5 days later received 1 × 106 radiolabelled macrophages by i.p. injection. Mice were kept on chow for 48 h until sacrifice.
- A. Different parameters in mice receiving scrambled (open bars) and anti-miR-33 (closed bars) treatment at the time of sacrifice. Notice the increase in bile recovery from the gallbladder.
- B. Percentage of total injected dpm recovered in the plasma at different time points after injection of radiolabelled cells.
- C-E. Percentage of total injected dpm recovered at the time of sacrifice in liver, bile from the gallbladder and faeces of the same mice. Data shown as mean ± SD; *p < 0.05; **p < 0.01.
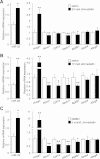
Figure 5. Statins induce miR-33 and reduce the mRNA expression of specific hepatic canalicular transporters
- A, B. C57BL/6 mice (n = 5) were gavaged daily with statins, and kept on chow diet. Samples were collected after 7 days, following an overnight fasting.
- C. HuH7 cells were cultured in quadruplicate for 48 h in DMEM supplemented with 2% lipoprotein-deficient serum in the presence or absence of simvastatin. Relative expression of specific genes shown as mean ± SD; *p < 0.05; **p < 0.01.
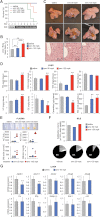
Figure 6. Simvastatin and lithogenic diet induce liver toxicity
C57BL/6 mice (n = 6) were gavaged daily with simvastatin and fed a lithogenic diet. Samples were collected after 7 days, or when mice appeared moribund.
- Survival is hampered by simvastatin in a dose-dependent manner.
- Severe hepatomegaly in mice dosed with 150 mpk simvastatin.
- Representative macroscopic appearance (upper and middle panels), and haematoxylin and eosin staining of paraffin-embedded sections (lower panels) from the same livers. Note the abnormally swollen cells and pale (i.e. steatotic) appearance of the livers in the 150 mpk group.
- Specific hepatic lipids as determined by ESI-MS, and normalized to tissue weight. FFA, free fatty acids; DAG, diacylglycerides; TAG, triacylglycerides; PC, phosphatidylcholine; UC, unesterified cholesterol; CE, cholesterol esters. **p < 0.01 versus saline.
- Representative samples of plasma, and levels of circulating alanine aminotransferase (ALT), aspartate aminotransferase (AST), bile acids and bilirubin.
- Bile was recovered from the gallbladder, pooled and the contents of phosphatidylcholine (PC), cholesterol (c) and bile acids (BA) determined with colorimetric kits.
- Relative expression of hepatic canalicular transporters (upper panel) and other genes involved in bile acid and sterol homeostasis (bottom panel) in samples from mice treated with 0 or 50 mpk simvastatin. Data shown as mean ± SD. **p < 0.01.
Figure 7. Silencing miR-33 rescues the liver damage induced by simvastatin and lithogenic diet
C57BL/6 mice (n = 12) were injected i.v. with 5 mpk scrambled or anti-miR-33 oligonucleotides for two consecutive days, and then treated as in Fig 5.
- Survival curves.
- Body weight, expressed as % compared to mass on the day of first injection.
- Representative macroscopic appearance of the livers, and haematoxylin and eosin staining of paraffin-embedded sections.
- Liver to total body mass ratios. **p < 0.01 versus moribund mice.
- Representative samples of plasma. Note the normalization in colour in the anti-miR-33 group.
- Amounts of specific hepatic lipids as determined by ESI-MS, normalized to tissue weight (n = 4). Acronyms for lipid classes as defined in Fig 5. **p < 0.01 versus scrambled.
- Relative expression of hepatic canalicular transporters (upper panel) and other genes involved in bile acid and sterol homeostasis (bottom panel) (n = 5 for scrambled treatment; n = 10 for anti-miR-33 treatment). Data shown as mean ± SEM. **p < 0.01 versus moribund scrambled mice; *p < 0.05 versus surviving scrambled mice.
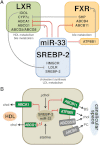
Figure 8. miR-33 limits the mobilization of sterols in hepatocytes through both the sinusoidal and the canalicular membrane
- miR-33 mediates the cross-talk between the SREBP-2, LXR and FXR pathways by directly modulating the expression of ATP8B1, ABCB11, ABCA1 and ABCG1 (* in mice, but not in humans). Data suggest that miR-33 might also indirectly affect the expression of ABCG5/8 (see main text for details). §Decreased ATP8B1 activity in human, but not mouse, hepatocytes results in PKC-dependent inactivating phosphorylation of FXR.
- During episodes of low intracellular cholesterol, or following treatment with statin drugs, miR-33 is transcriptionally induced and reduces the expression of sterol transporters.
Comment in
- Novel non-coding RNA-based therapeutic approaches to prevent statin-induced liver damage.
Bang C, Thum T. Bang C, et al. EMBO Mol Med. 2012 Sep;4(9):863-5. doi: 10.1002/emmm.201201565. Epub 2012 Aug 20. EMBO Mol Med. 2012. PMID: 22903913 Free PMC article. No abstract available.
Similar articles
- Activity of the bile salt export pump (ABCB11) is critically dependent on canalicular membrane cholesterol content.
Paulusma CC, de Waart DR, Kunne C, Mok KS, Elferink RP. Paulusma CC, et al. J Biol Chem. 2009 Apr 10;284(15):9947-54. doi: 10.1074/jbc.M808667200. Epub 2009 Feb 19. J Biol Chem. 2009. PMID: 19228692 Free PMC article. - Novel non-coding RNA-based therapeutic approaches to prevent statin-induced liver damage.
Bang C, Thum T. Bang C, et al. EMBO Mol Med. 2012 Sep;4(9):863-5. doi: 10.1002/emmm.201201565. Epub 2012 Aug 20. EMBO Mol Med. 2012. PMID: 22903913 Free PMC article. No abstract available. - Defective canalicular transport and toxicity of dietary ursodeoxycholic acid in the abcb11-/- mouse: transport and gene expression studies.
Wang R, Liu L, Sheps JA, Forrest D, Hofmann AF, Hagey LR, Ling V. Wang R, et al. Am J Physiol Gastrointest Liver Physiol. 2013 Aug 15;305(4):G286-94. doi: 10.1152/ajpgi.00082.2013. Epub 2013 Jun 13. Am J Physiol Gastrointest Liver Physiol. 2013. PMID: 23764895 - [Childhood cholestasis and bile transporters].
Hierro L, Jara P. Hierro L, et al. Gastroenterol Hepatol. 2005 Aug-Sep;28(7):388-95. doi: 10.1157/13077760. Gastroenterol Hepatol. 2005. PMID: 16137474 Review. Spanish. - Bile salt excretory pump: biology and pathobiology.
Suchy FJ, Ananthanarayanan M. Suchy FJ, et al. J Pediatr Gastroenterol Nutr. 2006 Jul;43 Suppl 1:S10-6. doi: 10.1097/01.mpg.0000226385.71859.5f. J Pediatr Gastroenterol Nutr. 2006. PMID: 16819395 Review.
Cited by
- Specific Disruption of Abca1 Targeting Largely Mimics the Effects of miR-33 Knockout on Macrophage Cholesterol Efflux and Atherosclerotic Plaque Development.
Price NL, Rotllan N, Zhang X, Canfrán-Duque A, Nottoli T, Suarez Y, Fernández-Hernando C. Price NL, et al. Circ Res. 2019 Mar 15;124(6):874-880. doi: 10.1161/CIRCRESAHA.118.314415. Circ Res. 2019. PMID: 30707082 Free PMC article. - miR-183 inhibits invasion of gastric cancer by targeting Ezrin.
Cao LL, Xie JW, Lin Y, Zheng CH, Li P, Wang JB, Lin JX, Lu J, Chen QY, Huang CM. Cao LL, et al. Int J Clin Exp Pathol. 2014 Aug 15;7(9):5582-94. eCollection 2014. Int J Clin Exp Pathol. 2014. PMID: 25337200 Free PMC article. - MicroRNAs involved in the lipid metabolism and their possible implications for atherosclerosis development and treatment.
Novák J, Bienertová-Vašků J, Kára T, Novák M. Novák J, et al. Mediators Inflamm. 2014;2014:275867. doi: 10.1155/2014/275867. Epub 2014 Apr 24. Mediators Inflamm. 2014. PMID: 24876669 Free PMC article. Review. - Perilipin-5 is regulated by statins and controls triglyceride contents in the hepatocyte.
Langhi C, Marquart TJ, Allen RM, Baldán A. Langhi C, et al. J Hepatol. 2014 Aug;61(2):358-65. doi: 10.1016/j.jhep.2014.04.009. Epub 2014 Apr 21. J Hepatol. 2014. PMID: 24768901 Free PMC article. - MicroRNAs in the Pathobiology and Therapy of Atherosclerosis.
Laffont B, Rayner KJ. Laffont B, et al. Can J Cardiol. 2017 Mar;33(3):313-324. doi: 10.1016/j.cjca.2017.01.001. Epub 2017 Jan 5. Can J Cardiol. 2017. PMID: 28232017 Free PMC article. Review.
References
- Abu Rajab M, Kaplan MM. Statins in primary biliary cirrhosis: are they safe. Dig Dis Sci. 2010;55:2086–2088. - PubMed
- Alvarez L, Jara P, Sanchez-Sabate E, Hierro L, Larrauri J, Diaz MC, Camarena C, De La Vega A, Frauca E, Lopez-Collazo E, et al. Reduced hepatic expression of Farnesoid X Receptor in hereditary cholestasis associated to mutation in ATP8B1. Hum Mol Genet. 2004;13:2451–2460. - PubMed
- Batey RG, Harvey M. Cholestasis associated with the use of pravastatin sodium. Med J Aust. 2002;176:561. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HL074214/HL/NHLBI NIH HHS/United States
- R01 HL074214/HL/NHLBI NIH HHS/United States
- R01 DK084434/DK/NIDDK NIH HHS/United States
- R21 HL098907/HL/NHLBI NIH HHS/United States
- HL098907/HL/NHLBI NIH HHS/United States
- HL107794/HL/NHLBI NIH HHS/United States
- RR019232/RR/NCRR NIH HHS/United States
- S10 RR019232/RR/NCRR NIH HHS/United States
- DK084434/DK/NIDDK NIH HHS/United States
- R01 HL107794/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases