Syntaxin-11, but not syntaxin-2 or syntaxin-4, is required for platelet secretion - PubMed (original) (raw)
Syntaxin-11, but not syntaxin-2 or syntaxin-4, is required for platelet secretion
Shaojing Ye et al. Blood. 2012.
Abstract
The platelet release reaction plays a critical role in thrombosis and contributes to the events that follow hemostasis. Previous studies have shown that platelet secretion is mediated by Soluble NSF Attachment Protein Receptor (SNARE) proteins from granule and plasma membranes. The SNAREs form transmembrane complexes that mediate membrane fusion and granule cargo release. Although VAMP-8 (v-SNARE) and SNAP-23 (a t-SNARE class) are important for platelet secretion, the identity of the functional syntaxin (another t-SNARE class) has been controversial. Previous studies using anti-syntaxin Abs in permeabilized platelets have suggested roles for both syntaxin-2 and syntaxin-4. In the present study, we tested these conclusions using platelets from syntaxin-knockout mouse strains and from a Familial Hemophagocytic Lymphohistiocytosis type 4 (FHL4) patient. Platelets from syntaxin-2 and syntaxin-4 single- or double-knockout mice had no secretion defect. Platelets from a FHL4 patient deficient in syntaxin-11 had a robust defect in agonist-induced secretion although their morphology, activation, and cargo levels appeared normal. Semiquantitative Western blotting showed that syntaxin-11 is the more abundant syntaxin in both human and murine platelets. Coimmunoprecipitation experiments showed that syntaxin-11 can form SNARE complexes with both VAMP-8 and SNAP-23. The results of the present study indicate that syntaxin-11, but not syntaxin-2 or syntaxin-4, is required for platelet exocytosis.
Figures
Figure 1
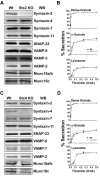
Deletion of syntaxin-2 (Stx2) or syntaxin-4 (Stx4) has no effect on platelet secretion. Platelet extracts (5.0 × 107 platelets/lane) were prepared from wild type (Wt) and syntaxin-2–knockout (Stx2 KO; A) or syntaxin-4–knockout (Stx4 KO; C) mice and the indicated proteins were detected by Western blotting. [3H]-serotonin–labeled platelets from Stx2 KO (B) or Stx4 KO (D; gray circle symbols) or Wt (black square symbols) were prepared as described in “Methods” and were stimulated with the indicated thrombin concentrations for 1 minute. Release of [3H]-serotonin from dense granules, PF4 from α-granules, and β-hexosaminidase from lysosomes was measured and the percentage of secretion was calculated. The data are the averages of triplicate measurements with the SD indicated.
Figure 2
Deletion of both syntaxin-2 and syntaxin-4 (Stx2/4) does not inhibit platelet secretion. (A) Platelet extracts (5.0 × 107 platelets/lane) were prepared from wild-type (Wt) and syntaxin-2 and syntaxin-4 double-knockout (Stx2/4 DKO) mice and the indicated proteins were detected by Western blotting. [3H]-serotonin–labeled platelets from Stx2/4 DKO (gray circle symbols) and Wt (black square symbols) were prepared as described in “Methods.” Release of [3H]-serotonin from dense granules, PF4 from α-granules, and β-hexosaminidase from lysosomes was measured as a thrombin-response curve (1-minute stimulation; B) or as a time course (0.05 U/mL of thrombin; C) and calculated as described in the legend to Figure 1.
Figure 3
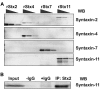
Syntaxin-11 is recognized by the syntaxin-2 polyclonal Ab. (A) Increasing amounts (2.5, 10, and 20 ng) of recombinant His6-tagged cytosolic domain of human syntaxin-2 (rStx2, lanes 1-3), human syntaxin-4 (rStx4, lanes 4-6), human syntaxin-7 (rStx7, lanes 7-9), and GST-tagged human syntaxin-11 (rStx11, lanes 10-12) were separated by SDS-PAGE and probed by Western blotting with the indicated Abs. (B) Human platelet extract was incubated with syntaxin-2–conjugated Sepharose beads. The specific bound proteins were eluted and subjected to Western blotting using anti–syntaxin-11 Ab. No Ab (−IgG) and nonspecific IgG (+IgG) controls were included.
Figure 4
Granule release is defective in syntaxin-11–deficient human platelets. Platelet extracts (5.0 × 107 platelets/lane) from control patients and an FHL4 patient were probed by Western blotting with the indicated Abs (A). [3H]-Serotonin labeled platelets from the FHL4 patient (gray circle symbols) and a normal control donor (black square symbols) were prepared as in “Methods” and stimulated with either the indicated thrombin concentration at room temperature for 1 minute (B) or 0.05 U/mL of thrombin for the indicated time points (C). Release of [3H]-serotonin from dense granules, PF4 from α-granules, and β-hexosaminidase from lysosomes was measured and calculated as described in the legend to Figure 1.
Figure 5
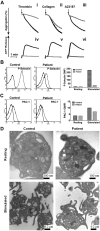
Depletion of syntaxin-11 affects aggregation, ATP release, and P-selectin exposure, but not integrin activation or platelet ultrastructure. (A) Aggregation (i-iii) and ATP release (iv-vi) were monitored concurrently in a lumi-aggregometer. Washed platelets from a control donor (black traces) and FHL4 patient (gray traces) were stimulated with thrombin (0.1 U/mL; i, iv), collagen (10 μg/mL; ii, v), and A23187 (100nM; iii, vi) for 2-3 minutes; B-C) Washed platelets from a control donor (Control) and FHL4 patient (Patient) were stimulated with 0.1 U/mL of thrombin for 1 minute and then incubated with FITC-conjugated anti–P-selectin (B) or FITC-conjugated PAC-1 (C) Abs. The reactions were stopped with hirudin and the fluorescent intensities were measured by flow cytometry. The data were plotted as a histogram (left panels) and as the geometric mean fluorescence intensity (GMFI; right panels). Because of limited samples, the experiments in panels B and C were done only once. (D) Washed platelets from a control donor (Control) and FHL4 patient (Patient) were kept resting with 1 ng/mL of PGI2 (i-ii) or stimulated with 0.1 U/mL of thrombin (iii-iv) for 5 minutes. The platelets were fixed and processed for electron microscopic analysis as described in “Methods.” The samples were analyzed with a transmission electron microscope and images were obtained using Gatan software. The scale bars are indicated.
Figure 6
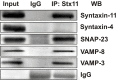
Syntaxin-11 is associated with functionally relevant platelet SNAREs. Platelet extracts (Input) from resting human platelets were prepared by solubilization with 1% Triton X-100. After clarification, platelet extracts were incubated with syntaxin-11 polyclonal Ab or IgG control for 3 hours at 4°C. Immune complexes were recovered with protein A Sepharose. The bound proteins were eluted and separated by SDS-PAGE, followed by Western blotting with the indicated Abs.
Comment in
- SNARing platelet granule secretion.
Marks MS. Marks MS. Blood. 2012 Sep 20;120(12):2355-7. doi: 10.1182/blood-2012-07-442756. Blood. 2012. PMID: 22996656
Similar articles
- Munc18b/STXBP2 is required for platelet secretion.
Al Hawas R, Ren Q, Ye S, Karim ZA, Filipovich AH, Whiteheart SW. Al Hawas R, et al. Blood. 2012 Sep 20;120(12):2493-500. doi: 10.1182/blood-2012-05-430629. Epub 2012 Jul 12. Blood. 2012. PMID: 22791290 Free PMC article. - SNARing platelet granule secretion.
Marks MS. Marks MS. Blood. 2012 Sep 20;120(12):2355-7. doi: 10.1182/blood-2012-07-442756. Blood. 2012. PMID: 22996656 - Vesicle-associated membrane protein 3 (VAMP-3) and VAMP-8 are present in human platelets and are required for granule secretion.
Polgár J, Chung SH, Reed GL. Polgár J, et al. Blood. 2002 Aug 1;100(3):1081-3. doi: 10.1182/blood.v100.3.1081. Blood. 2002. PMID: 12130530 - A unique SNARE machinery for exocytosis of cytotoxic granules and platelets granules.
Tang BL. Tang BL. Mol Membr Biol. 2015;32(4):120-6. doi: 10.3109/09687688.2015.1079934. Mol Membr Biol. 2015. PMID: 26508555 Review. - The nuts and bolts of the platelet release reaction.
Joshi S, Whiteheart SW. Joshi S, et al. Platelets. 2017 Mar;28(2):129-137. doi: 10.1080/09537104.2016.1240768. Epub 2016 Nov 16. Platelets. 2017. PMID: 27848265 Free PMC article. Review.
Cited by
- Sequential and compartmentalized action of Rabs, SNAREs, and MAL in the apical delivery of fusiform vesicles in urothelial umbrella cells.
Wankel B, Ouyang J, Guo X, Hadjiolova K, Miller J, Liao Y, Tham DK, Romih R, Andrade LR, Gumper I, Simon JP, Sachdeva R, Tolmachova T, Seabra MC, Fukuda M, Schaeren-Wiemers N, Hong WJ, Sabatini DD, Wu XR, Kong X, Kreibich G, Rindler MJ, Sun TT. Wankel B, et al. Mol Biol Cell. 2016 May 15;27(10):1621-34. doi: 10.1091/mbc.E15-04-0230. Epub 2016 Mar 23. Mol Biol Cell. 2016. PMID: 27009205 Free PMC article. - Enrichment of the exocytosis protein STX4 in skeletal muscle remediates peripheral insulin resistance and alters mitochondrial dynamics via Drp1.
Merz KE, Hwang J, Zhou C, Veluthakal R, McCown EM, Hamilton A, Oh E, Dai W, Fueger PT, Jiang L, Huss JM, Thurmond DC. Merz KE, et al. Nat Commun. 2022 Jan 20;13(1):424. doi: 10.1038/s41467-022-28061-w. Nat Commun. 2022. PMID: 35058456 Free PMC article. - Platelets and cancer: a casual or causal relationship: revisited.
Menter DG, Tucker SC, Kopetz S, Sood AK, Crissman JD, Honn KV. Menter DG, et al. Cancer Metastasis Rev. 2014 Mar;33(1):231-69. doi: 10.1007/s10555-014-9498-0. Cancer Metastasis Rev. 2014. PMID: 24696047 Free PMC article. Review. - α-Synuclein is the major platelet isoform but is dispensable for activation, secretion, and thrombosis.
Smith AN, Joshi S, Chanzu H, Alfar HR, Shravani Prakhya K, Whiteheart SW. Smith AN, et al. Platelets. 2023 Dec;34(1):2267147. doi: 10.1080/09537104.2023.2267147. Epub 2023 Nov 5. Platelets. 2023. PMID: 37927048 Free PMC article. - Role of Munc13-4 as a Ca2+-dependent tether during platelet secretion.
Chicka MC, Ren Q, Richards D, Hellman LM, Zhang J, Fried MG, Whiteheart SW. Chicka MC, et al. Biochem J. 2016 Mar 1;473(5):627-39. doi: 10.1042/BJ20151150. Epub 2015 Dec 4. Biochem J. 2016. PMID: 26637270 Free PMC article.
References
- Reed GL. Platelet secretory mechanisms. Semin Thromb Hemost. 2004;30(4):441–450. - PubMed
- Cattaneo M. Inherited platelet-based bleeding disorders. J Thromb Haemost. 2003;1(7):1628–1636. - PubMed
- Polgár J, Chung SH, Reed GL. Vesicle-associated membrane protein 3 (VAMP-3) and VAMP-8 are present in human platelets and are required for granule secretion. Blood. 2002;100(3):1081–1083. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HL56652/HL/NHLBI NIH HHS/United States
- R21 AI079759/AI/NIAID NIH HHS/United States
- AI079759/AI/NIAID NIH HHS/United States
- AI076746/AI/NIAID NIH HHS/United States
- R01 HL056652/HL/NHLBI NIH HHS/United States
- HL082193/HL/NHLBI NIH HHS/United States
- S10 RR026827/RR/NCRR NIH HHS/United States
- L40 HL082193/HL/NHLBI NIH HHS/United States
- R21 AI076746/AI/NIAID NIH HHS/United States
- P20 RR020171/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases