Translational control in cancer etiology - PubMed (original) (raw)
Review
Translational control in cancer etiology
Davide Ruggero. Cold Spring Harb Perspect Biol. 2013.
Erratum in
- Cold Spring Harb Perspect Biol. 2012 Nov;4(11). doi:10.1101/cshperspect.a015891
Abstract
The link between perturbations in translational control and cancer etiology is becoming a primary focus in cancer research. It has now been established that genetic alterations in several components of the translational apparatus underlie spontaneous cancers as well as an entire class of inherited syndromes known as "ribosomopathies" associated with increased cancer susceptibility. These discoveries have illuminated the importance of deregulations in translational control to very specific cellular processes that contribute to cancer etiology. In addition, a growing body of evidence supports the view that deregulation of translational control is a common mechanism by which diverse oncogenic pathways promote cellular transformation and tumor development. Indeed, activation of these key oncogenic pathways induces rapid and dramatic translational reprogramming both by increasing overall protein synthesis and by modulating specific mRNA networks. These translational changes promote cellular transformation, impacting almost every phase of tumor development. This paradigm represents a new frontier in the multihit model of cancer formation and offers significant promise for innovative cancer therapies. Current research, in conjunction with cutting edge technologies, will further enable us to explore novel mechanisms of translational control, functionally identify translationally controlled mRNA groups, and unravel their impact on cellular transformation and tumorigenesis.
Figures
Figure 1.
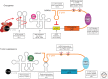
Deregulations in translational control contribute to each step of cellular transformation and tumor progression. (A) Upon receiving an oncogenic insult (lightning bolt) such as Myc overexpression or PI3K hyperactivation, ribosome biogenesis and global protein synthesis are augmented resulting in increased cell size coupled to cell division. Upon this oncogenic stress, cells initiate a tumor-suppressive response associated with increased internal ribosome entry site (IRES)-mediated translation, leading to cell-cycle arrest and senescence. To overcome the barrier of oncogene-induced senescence (OIS), cells acquire additional mutations known as secondary hits (lightning bolt). One such mechanism to overcome tumor-suppressive checkpoints such as OIS is the promotion of genome instability by decreasing IRES-dependent translation of the CDK11/p58 tumor suppressor. (B) Once established, a primary tumor will undergo unrestrained growth, which triggers a stress response including depletion of oxygen and essential nutrients from the core of the tumor. Lack of key nutrients such as growth factors often leads to increased apoptosis (skulls). Tumor cells up-regulate cap-independent translation of antiapoptotic factors (e.g., Bcl-2 and XIAP) as a mechanism to promote survival (blocked skulls). To bypass stress caused by low levels of oxygen in the tumor, cells induce cap-independent translation of neoangiogenesis promoting factors such as vascular endothelial growth factor (VEGF). Primary tumors metastasize to secondary organs. Epithelial-to-mesenchymal transition (EMT) facilitates metastatic dissemination; specifically, cancer cells lose their epithelial characteristics (yellow cells) and acquire mesenchymal traits (blue cells) including enhanced mobility and invasiveness. Multiple stages of metastatic cell dissemination include degradation of the basement membrane, intravasation into the circulatory system, and extravasation at the distal site. eIF4E regulates the translation of an invasive messenger RNA (mRNA) signature (i.e., YB-1, MTA1, vimentin, and CD44) that promotes metastasis formation. Increased translation of prometastatic factors localized at the leading edge of the tumor’s invasive front may facilitate metastasis colonization.
Figure 2.
Oncogenes and tumor suppressors are exquisitely regulated at the translational level through specific regulatory elements in their mRNAs. Depictions of _cis_-regulatory elements present in oncogene (top) and tumor suppressor (bottom) mRNAs including key cell-cycle and survival factors. Examples of regulated mRNAs are given (boxes). The cap-binding protein eIF4E binds the 5′ cap of mRNAs. Increased eIF4E activity recruits eIF4G and the eIF4A helicase to unwind (red arrow) structured elements in the 5′ UTR of poorly translated mRNAs, increasing the expression of many growth promoting and prosurvival genes such as Mcl-1. IRES elements are structures that also direct translational regulation by promoting cap-independent protein synthesis of both tumor suppressors (p53) and prosurvival factors (XIAP) during different stages of tumor development (see Fig. 1). IRES _trans_-acting factors (ITAFs) modulate translational regulation directed by specific IRES elements. Upstream open reading frames (uORF) are present in select mRNAs (including growth promoting factors such as Mdm2 and Her-2) and inhibit translation initiation by preventing the ribosome from scanning to the start codon. Additionally, a region in the 3′ UTR of the Her-2 mRNA can inhibit uORF-mediated repression of translation. 3′ UTRs contain many distinct elements that interact with RNA-binding proteins (RBPs) and microRNAs (miRNAs) to promote or inhibit translation. One interesting example is the regulation of p53 translation mediated by base-pairing interactions between elements in the 5′ and 3′ UTRs of p53.
Figure 3.
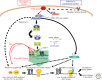
Oncogenic signals regulate each stage of translation. Oncogenic stimuli (red) such as PI3K-AKT-mTOR, Myc, and Ras promote protein synthesis by coordinating the regulation of ribosome biogenesis, translation initiation, and translation elongation. The PI3K-AKT-mTOR signaling pathway promotes ribosome biogenesis through both enhanced rRNA synthesis and enhanced ribosomal protein production (Hannan et al. 2003; Martin et al. 2004; Mayer et al. 2004). This signaling pathway stimulates translation initiation predominantly through mTORC1-dependent hyperactivation of eIF4E. In the absence of signaling, hypophosphorylated 4E-BPs bind to and inhibit eIF4E, blocking its ability to interact with eIF4G. PI3K-AKT signaling activates mTORC1, initiating a series of phosphorylations that release 4E-BPs from eIF4E. This allows for eIF4G binding to eIF4E and the subsequent recruitment of the 40S ribosomal subunit. Furthermore, S6K1/2 downstream mammalian target of rapamycin (mTOR) affects the efficiency of translation initiation and elongation (Ma and Blenis 2009). In addition, Ras-MAP kinase signaling up-regulates eIF4E activation via phosphorylation at serine 209. Myc promotes protein synthesis by increasing the transcription of multiple translational components including eIF4E mRNA. Together, these oncogenic stimuli regulate the multiple stages of translation to drive both global changes in protein synthesis as well as selective changes in the translation of specific mRNAs. Multiple approaches (blue) are used to therapeutically target the translational apparatus including rapamycin, ATP-active site inhibitors of mTOR, MNK1/2 kinase inhibitors, 4EGI-1, and eIF4E antisense oligonucleotides (ASO).
Similar articles
- The cancerous translation apparatus.
Stumpf CR, Ruggero D. Stumpf CR, et al. Curr Opin Genet Dev. 2011 Aug;21(4):474-83. doi: 10.1016/j.gde.2011.03.007. Epub 2011 May 3. Curr Opin Genet Dev. 2011. PMID: 21543223 Free PMC article. Review. - Translational control in cancer.
Silvera D, Formenti SC, Schneider RJ. Silvera D, et al. Nat Rev Cancer. 2010 Apr;10(4):254-66. doi: 10.1038/nrc2824. Nat Rev Cancer. 2010. PMID: 20332778 Review. - Translation initiation and its relevance in colorectal cancer.
Minnee E, Faller WJ. Minnee E, et al. FEBS J. 2021 Dec;288(23):6635-6651. doi: 10.1111/febs.15690. Epub 2021 Jan 24. FEBS J. 2021. PMID: 33382175 Free PMC article. Review. - Oncogenic AKTivation of translation as a therapeutic target.
Hsieh AC, Truitt ML, Ruggero D. Hsieh AC, et al. Br J Cancer. 2011 Jul 26;105(3):329-36. doi: 10.1038/bjc.2011.241. Epub 2011 Jul 19. Br J Cancer. 2011. PMID: 21772331 Free PMC article. Review. - The Akt of translational control.
Ruggero D, Sonenberg N. Ruggero D, et al. Oncogene. 2005 Nov 14;24(50):7426-34. doi: 10.1038/sj.onc.1209098. Oncogene. 2005. PMID: 16288289 Review.
Cited by
- The Performance Comparison of Gene Co-expression Networks of Breast and Prostate Cancer using Different Selection Criteria.
Cingiz MÖ, Biricik G, Diri B. Cingiz MÖ, et al. Interdiscip Sci. 2021 Sep;13(3):500-510. doi: 10.1007/s12539-021-00440-9. Epub 2021 May 18. Interdiscip Sci. 2021. PMID: 34003445 - Ribosome heterogeneity in tumorigenesis: the rRNA point of view.
Marcel V, Catez F, Diaz JJ. Marcel V, et al. Mol Cell Oncol. 2015 Mar 24;2(3):e983755. doi: 10.4161/23723556.2014.983755. eCollection 2015 Jul-Sep. Mol Cell Oncol. 2015. PMID: 27305893 Free PMC article. - Paip1 overexpression is involved in the progression of gastric cancer and predicts shorter survival of diagnosed patients.
Wang Q, Han A, Chen L, Sun J, Lin Z, Zhang X, Ren X. Wang Q, et al. Onco Targets Ther. 2019 Aug 16;12:6565-6576. doi: 10.2147/OTT.S202698. eCollection 2019. Onco Targets Ther. 2019. PMID: 31496746 Free PMC article. - Identification and targeting of novel CDK9 complexes in acute myeloid leukemia.
Beauchamp EM, Abedin SM, Radecki SG, Fischietti M, Arslan AD, Blyth GT, Yang A, Lantz C, Nelson A, Goo YA, Akpan I, Eklund EA, Frankfurt O, Fish EN, Thomas PM, Altman JK, Platanias LC. Beauchamp EM, et al. Blood. 2019 Mar 14;133(11):1171-1185. doi: 10.1182/blood-2018-08-870089. Epub 2018 Dec 26. Blood. 2019. PMID: 30587525 Free PMC article. - The Helix-Loop-Helix motif of human EIF3A regulates translation of proliferative cellular mRNAs.
Volegova MP, Hermosillo C, Cate JHD. Volegova MP, et al. PLoS One. 2023 Sep 28;18(9):e0292080. doi: 10.1371/journal.pone.0292080. eCollection 2023. PLoS One. 2023. PMID: 37768948 Free PMC article.
References
- Abraham N, Stojdl DF, Duncan PI, Methot N, Ishii T, Dube M, Vanderhyden BC, Atkins HL, Gray DA, McBurney MW, et al. 1999. Characterization of transgenic mice with targeted disruption of the catalytic domain of the double-stranded RNA-dependent protein kinase, PKR. J Biol Chem 274: 5953–5962 - PubMed
- Ahlemann M, Zeidler R, Lang S, Mack B, Munz M, Gires O 2006. Carcinoma-associated eIF3i overexpression facilitates mTOR-dependent growth transformation. Mol Carcinog 45: 957–967 - PubMed
- Anand N, Murthy S, Amann G, Wernick M, Porter LA, Cukier IH, Collins C, Gray JW, Diebold J, Demetrick DJ, et al. 2002. Protein elongation factor EEF1A2 is a putative oncogene in ovarian cancer. Nat Genet 31: 301–305 - PubMed
- Arabi A, Rustum C, Hallberg E, Wright AP 2003. Accumulation of c-Myc and proteasomes at the nucleoli of cells containing elevated c-Myc protein levels. J Cell Sci 116: 1707–1717 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources