Acetylation-dependent regulation of Skp2 function - PubMed (original) (raw)
. 2012 Jul 6;150(1):179-93.
doi: 10.1016/j.cell.2012.05.038.
Daming Gao, Lydia W S Finley, Wen Yang, Lixin Wan, Hidefumi Fukushima, Y Rebecca Chin, Bo Zhai, Shavali Shaik, Alan W Lau, Zhiwei Wang, Steven P Gygi, Keiko Nakayama, Julie Teruya-Feldstein, Alex Toker, Marcia C Haigis, Pier Paolo Pandolfi, Wenyi Wei
Affiliations
- PMID: 22770219
- PMCID: PMC3595190
- DOI: 10.1016/j.cell.2012.05.038
Acetylation-dependent regulation of Skp2 function
Hiroyuki Inuzuka et al. Cell. 2012.
Abstract
Aberrant Skp2 signaling has been implicated as a driving event in tumorigenesis. Although the underlying molecular mechanisms remain elusive, cytoplasmic Skp2 correlates with more aggressive forms of breast and prostate cancers. Here, we report that Skp2 is acetylated by p300 at K68 and K71, which is a process that can be antagonized by the SIRT3 deacetylase. Inactivation of SIRT3 leads to elevated Skp2 acetylation, which leads to increased Skp2 stability through impairment of the Cdh1-mediated proteolysis pathway. As a result, Skp2 oncogenic function is increased, whereby cells expressing an acetylation-mimetic mutant display enhanced cellular proliferation and tumorigenesis in vivo. Moreover, acetylation of Skp2 in the nuclear localization signal (NLS) promotes its cytoplasmic retention, and cytoplasmic Skp2 enhances cellular migration through ubiquitination and destruction of E-cadherin. Thus, our study identifies an acetylation-dependent regulatory mechanism governing Skp2 oncogenic function and provides insight into how cytoplasmic Skp2 controls cellular migration.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures
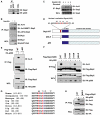
Figure 1. Skp2 Is Acetylated by p300 at K68 and K71
(A) Immunoblot (IB) analysis of 293T whole-cell lysates (WCL) and anti-p300 immunoprecipitates (IP). Rabbit IgG was used as a negative control for the immunoprecipitation procedure. (B and C) IB analysis of WCL derived from HeLa cells that were serum starved for 24 hr and then collected after 1 hr following addition of insulin to activate the PI3K/Akt signaling pathway. Cells were pretreated with TSA (2 μM) and NAM (10 mM) for 1 hr before the addition of insulin. (D) Schematic representation of the Skp2 deletion mutants used in (E) and (F). (E and F) IB analysis of WCL and anti-Flag IP derived from 293T cells transfected with HA-p300 and various Flag-human Skp2 constructs. (G) Sequence alignment of the putative acetylation sites K68 and K71 in Skp2 from different species. (H) IB analysis of WCL and anti-Flag IP derived from 293T cells transfected with HA-p300 and the indicated Flag-mouse Skp2 constructs. See also Figure S1.
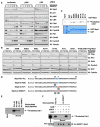
Figure 2. SIRT3 Interacts with and Deacetylates Skp2
(A) Autoradiography of 35S-labeled Sirtuins bound to HA-Skp2 immunoprecipitated from the transfected 293T cells. Empty vector (EV) transfection was used as a negative control. (B) IB analysis of WCL and anti-Skp2 IP derived from HeLa cells that were infected with the indicated lentiviral vector. Anti-HA IgG was used as a negative control for the immunoprecipitation procedure. (C) IB analysis of WCL and anti-Flag IP derived from 293T cells transfected with HA-p300, Flag-Skp2, and the various Flag-SIRT3 constructs. (D) Schematic representation of the various biotinylated peptides used in (E). Where indicated, the K68 and/or K71 residue is acetylated. (E) 2 μg of indicated peptides were incubated in the presence or absence of recombinant SIRT3 at 37°C for 2 hr. Where indicated, 1.5 mM NAD or 20 mM NAM were added into the reaction. The indicated amount of peptides after reaction was spotted on nitrocellulose membrane and immunoblotted with the Ac-K68K71-Skp2 antibody. (F) IB analysis of WCL and anti-Skp2 IP derived from HeLa cells that were infected with the indicated lentiviral vector. Before Skp2 immunoprecipitation, the indicated cells were serum starved for 24 hr and then collected at the indicated time periods following the addition of insulin. Cells were pretreated with TSA (2 μM) for 1 hr before the addition of insulin. (G–I) IB analysis of WCL and anti-Skp2 IP derived from WT, _SIRT3_−/− (G–H), or _SIRT4_−/− (I) MEFs. Before Skp2 immunoprecipitation, the indicated cells were serum starved for 24 hr and then collected 2 hr following addition of insulin. Cells were pretreated with TSA (2 μM) for 1 hr before the addition of insulin. Where indicated, 10 mM ROS inhibitor NAC were added for 16 hr before harvesting for IP (H). (J) IB analysis of WCL and anti-Skp2 IP derived from HeLa cells that were infected with the indicated lentiviral vector. Before Skp2 immunoprecipitation, the indicated cells were serum starved for 24 hr and then collected at the indicated time periods following the addition of insulin. Cells were pretreated with TSA (2 mM) for 1 hr before addition of insulin. (K) IB analysis of WCL derived from WT or _SIRT3_−/− MEFs. (L and M) Representative images of SIRT3 and Skp2 expression in breast tumor cells as assessed by immunohistochemistry (L). Both Skp2 and SIRT3 levels were classified as low, medium, or high based on the intensities of the IHC staining, and the percentages of patients classified in each category are depicted in the histogram in (M). (N) Growth curves for the xenograft experiments with the indicated tumor cells that were inoculated subcutaneously. In each flank of nine nude mice, 8 × 106 cells were injected. The visible tumors were measured at the indicated days. Error bars represent ±SEM, and *p < 0.05 (Student’s t test). See also Figure S2.
Figure 3. p300-Dependent Acetylation of Skp2 Impairs Cdh1-Mediated Skp2 Proteolysis Pathway
(A) IB analysis of HeLa cells transfected with the indicated siRNA oligos after synchronization with nocodazole and release at the indicated time periods. (B) IB analysis of HeLa cells transfected with limited amount of the indicated Flag-Skp2 constructs, along with a green fluorescent protein (GFP) as a transfection control. HeLa cells were synchronized in the M phase with nocodazole and then released into G1 for the indicated time periods. (C) Autoradiography of 35S-labeled Cdh1 bound to the indicated GST fusion proteins. (D) Schematic representation of the various biotinylated peptides used in Figures 3E, 3F, 5D, and 5E, which are derived from the Cdh1-interaction motif of Skp2. Where indicated, the K68 and/or K71 residue is acetylated. (E) Autoradiography of 35S-labeled Cdh1 bound to the indicated biotinylated peptides. (F) Autoradiography of 35S-labeled Cdh1 bound to the indicated biotinylated peptides that have been subject to SIRT3 in vitro deacetylation assays as described in Figure 2E. See also Figure S3.
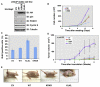
Figure 4. Acetylation of Skp2 Positively Regulates Skp2 Oncogenic Functions
(A) IB analysis of LNCaP cell lines stably transfected with the indicated HA-Skp2 constructs. (B) Cell growth curves of the various LNCaP cell lines stably expressing the indicated HA-Skp2 constructs. Results were presented as mean ±SD from three independent experiments. (C) Various LNCaP cell lines stably expressing the indicated HA-Skp2 constructs were pulsed with BrdU for 30 min, and the BrdU incorporation rate was measured. Results were presented as mean ±SD from three independent experiments. (D and E) LNCaP cells stably transfected with the indicated HA-Skp2 constructs (with empty vector as a negative control) were injected subcutaneously into nude mice (n = 5 for each group) and examined over time for in vivo tumorigenesis. Pictures in (E) were taken 6 weeks after injection. Results were presented as mean ±SD and *p < 0.05 (Student’s t test). See also Figure S4.
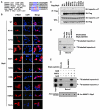
Figure 5. Acetylation of Skp2 by p300 Promotes Skp2 Cytoplasmic Localization
(A) Sequence alignment of the Skp2 NLS with p21, p27, and FOXO1 NLS. (B) Immunofluorescence and DAPI staining of 293T cells transfected with the indicated Flag-Skp2 constructs. (C) IB analysis of WCL and anti-Flag IP derived from 293T cells transfected with the indicated Flag-Skp2 constructs. (D) Autoradiography of 35S-labeled importin α1 or importin α5 bound to the indicated biotinylated peptides. (E) Autoradiography of 35S-labeled importin α5 or importin α7 bound to the indicated biotinylated peptides, which have been subject to SIRT3 in vitro deacetylation assays as described in Figure 2E. See also Figure S5.
Figure 6. Cytosolic Skp2 Plays a Critical Role in Cellular Migration
(A and B) Skp2 is required for cell migration. WT and _Skp2_−/− MEFs were plated for in vitro wound healing assays (A). Results in (A) were quantified in (B) and presented as mean ±SD from three independent experiments. (C) WT and _Skp2_−/− MEFs were infected with the indicated viral constructs before plating for transwell assay. The results were quantified and presented as mean ±SD from three independent experiments. (D) IB analysis of WT and _Skp2_−/− MEFs, synchronized by serum starvation for 72 hr and then released by readdition of serum for the indicated periods. (E and F) IB analysis of normal human fibroblasts (E) or SKOV3 epithelial cancer cell line (F) infected with the indicated lentiviral shRNA vectors. (G) IB analysis of WT and _Skp2_−/− MEFs infected with the indicated viral constructs. (H) IB analysis of DU145 cells infected with the indicated viral constructs. (I and J) IB analysis of MCF7 (I) or ZR75 epithelial cancer cell line (J) infected with the indicated lentiviral shRNA vectors. (K) IB analysis of MCF7 WCL and anti-E-cadherin IP. Anti-HA IgG was used as a negative control for the immunoprecipitation procedure. (L) IB analysis of WCL of HeLa cells transfected with the indicated plasmids. Cells were treated 20 hr posttransfection with the proteasome inhibitor MG132 overnight before harvesting. (M and N) IB analysis of WCL derived from DU145 (M) or SKOV3 (N) cells treated with 10 μM MG132 for 12 hr before harvesting. (O and P) Representative images of E-cadherin and Skp2 expression in breast tumor cells as assessed by immunohistochemistry (O). Both Skp2 and E-cadherin levels were classified as low, medium, or high based on the intensities of the IHC staining, and the percentages of patients classified in each category are depicted in the histogram in (P). See also Figure S6.
Figure 7. Skp2 Promotes the Ubiquitination and Destruction of E-Cadherin in a CKI-Dependent Manner
(A) Sequence alignment of the putative CKI phosphorylation sites in Skp2 from various species. (B) Indicated GST-fusion proteins were incubated with CKIδ and [γ-32P]ATP. The kinase reaction products were resolved by SDS-PAGE, and phosphorylation was detected by autoradiography. (C and D) Autoradiography of 35S-labeled Skp2 bound to the indicated GST-fusion proteins. Where indicated, GST-fusion proteins were pretreated with CKId before pull-down assays were performed. (E) IB analysis of WCL and anti-Myc IP derived from HeLa cells transfected with the indicated Myc-E-cadherin constructs. (F) IB analysis of WCL from HeLa cells transfected with the indicated plasmids. (G) SCFSkp2 E3 ligase complex promotes E-cadherin ubiquitination in vitro. Where indicated, GST-E-cadherin proteins were pretreated with CKI before the in vitro ubiquitination assays. (H) Proposed model for how acetylation of Skp2, which subsequently regulates Skp2 stability and cellular localization to influence its oncogenic functions, is governed by both p300 and SIRT3. See also Figure S7.
Similar articles
- Pharmacological inhibition of the SKP2/p300 signaling axis restricts castration-resistant prostate cancer.
Rezaeian AH, Phan LM, Zhou X, Wei W, Inuzuka H. Rezaeian AH, et al. Neoplasia. 2023 Apr;38:100890. doi: 10.1016/j.neo.2023.100890. Epub 2023 Mar 3. Neoplasia. 2023. PMID: 36871351 Free PMC article. - Identification of acetylation-dependent regulatory mechanisms that govern the oncogenic functions of Skp2.
Wang Z, Inuzuka H, Zhong J, Liu P, Sarkar FH, Sun Y, Wei W. Wang Z, et al. Oncotarget. 2012 Nov;3(11):1294-300. doi: 10.18632/oncotarget.740. Oncotarget. 2012. PMID: 23230084 Free PMC article. - Phosphorylation by Akt1 promotes cytoplasmic localization of Skp2 and impairs APCCdh1-mediated Skp2 destruction.
Gao D, Inuzuka H, Tseng A, Chin RY, Toker A, Wei W. Gao D, et al. Nat Cell Biol. 2009 Apr;11(4):397-408. doi: 10.1038/ncb1847. Epub 2009 Mar 8. Nat Cell Biol. 2009. PMID: 19270695 Free PMC article. - Regulation of Skp2 expression and activity and its role in cancer progression.
Chan CH, Lee SW, Wang J, Lin HK. Chan CH, et al. ScientificWorldJournal. 2010 Jun 1;10:1001-15. doi: 10.1100/tsw.2010.89. ScientificWorldJournal. 2010. PMID: 20526532 Free PMC article. Review. - Novel roles of Skp2 E3 ligase in cellular senescence, cancer progression, and metastasis.
Wang G, Chan CH, Gao Y, Lin HK. Wang G, et al. Chin J Cancer. 2012 Apr;31(4):169-77. doi: 10.5732/cjc.011.10319. Epub 2011 Dec 23. Chin J Cancer. 2012. PMID: 22200179 Free PMC article. Review.
Cited by
- PSEN1 is associated with colon cancer development via potential influences on PD-L1 nuclear translocation and tumor-immune interactions.
Wei W, Zhang Y. Wei W, et al. Front Immunol. 2022 Aug 17;13:927474. doi: 10.3389/fimmu.2022.927474. eCollection 2022. Front Immunol. 2022. PMID: 36059511 Free PMC article. - Mediator kinase module and human tumorigenesis.
Clark AD, Oldenbroek M, Boyer TG. Clark AD, et al. Crit Rev Biochem Mol Biol. 2015;50(5):393-426. doi: 10.3109/10409238.2015.1064854. Epub 2015 Jul 16. Crit Rev Biochem Mol Biol. 2015. PMID: 26182352 Free PMC article. Review. - Pharmacological inhibition of the SKP2/p300 signaling axis restricts castration-resistant prostate cancer.
Rezaeian AH, Phan LM, Zhou X, Wei W, Inuzuka H. Rezaeian AH, et al. Neoplasia. 2023 Apr;38:100890. doi: 10.1016/j.neo.2023.100890. Epub 2023 Mar 3. Neoplasia. 2023. PMID: 36871351 Free PMC article. - Histone Deacetylase Inhibitors Increase p27(Kip1) by Affecting Its Ubiquitin-Dependent Degradation through Skp2 Downregulation.
Borriello A, Naviglio S, Bencivenga D, Caldarelli I, Tramontano A, Speranza MC, Stampone E, Sapio L, Negri A, Oliva A, Sinisi AA, Spina A, Della Ragione F. Borriello A, et al. Oxid Med Cell Longev. 2016;2016:2481865. doi: 10.1155/2016/2481865. Epub 2015 Nov 22. Oxid Med Cell Longev. 2016. PMID: 26682002 Free PMC article. - The β-TrCP-FBXW2-SKP2 axis regulates lung cancer cell growth with FBXW2 acting as a tumour suppressor.
Xu J, Zhou W, Yang F, Chen G, Li H, Zhao Y, Liu P, Li H, Tan M, Xiong X, Sun Y. Xu J, et al. Nat Commun. 2017 Jan 16;8:14002. doi: 10.1038/ncomms14002. Nat Commun. 2017. PMID: 28090088 Free PMC article.
References
- Balasubramanyam K, Altaf M, Varier RA, Swaminathan V, Ravindran A, Sadhale PP, Kundu TK. Polyisoprenylated benzophenone, garcinol, a natural histone acetyltransferase inhibitor, represses chromatin transcription and alters global gene expression. J. Biol. Chem. 2004;279:33716–33726. - PubMed
- Bao J, Lu Z, Joseph JJ, Carabenciov D, Dimond CC, Pang L, Samsel L, McCoy JP, Jr., Leclerc J, Nguyen P, et al. Characterization of the murine SIRT3 mitochondrial localization sequence and comparison of mitochondrial enrichment and deacetylase activity of long and short SIRT3 isoforms. J. Cell. Biochem. 2010;110:238–247. - PMC - PubMed
- Bashir T, Dorrello NV, Amador V, Guardavaccaro D, Pagano M. Control of the SCF(Skp2-Cks1) ubiquitin ligase by the APC/C(Cdh1) ubiquitin ligase. Nature. 2004;428:190–193. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AG041218/AG/NIA NIH HHS/United States
- GM089763/GM/NIGMS NIH HHS/United States
- R01 GM089763/GM/NIGMS NIH HHS/United States
- CA122099/CA/NCI NIH HHS/United States
- R01 CA122099/CA/NCI NIH HHS/United States
- GM094777/GM/NIGMS NIH HHS/United States
- R01 GM094777/GM/NIGMS NIH HHS/United States
- K01 AG041218/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous