Structural mechanism for alteration of collagen gel mechanics by glutaraldehyde crosslinking - PubMed (original) (raw)
Structural mechanism for alteration of collagen gel mechanics by glutaraldehyde crosslinking
Preethi L Chandran et al. Connect Tissue Res. 2012.
Abstract
Soft collagenous tissues that are loaded in vivo undergo crosslinking during aging and wound healing. Bioprosthetic tissues implanted in vivo are also commonly crosslinked with glutaraldehyde (GA). While crosslinking changes the mechanical properties of the tissue, the nature of the mechanical changes and the underlying microstructural mechanism are poorly understood. In this study, a combined mechanical, biochemical and simulation approach was employed to identify the microstructural mechanism by which crosslinking alters mechanical properties. The model collagenous tissue used was an anisotropic cell-compacted collagen gel, and the model crosslinking agent was monomeric GA. The collagen gels were incrementally crosslinked by either increasing the GA concentration or increasing the crosslinking time. In biaxial loading experiments, increased crosslinking produced (1) decreased strain response to a small equibiaxial preload, with little change in response to subsequent loading and (2) decreased coupling between the fiber and cross-fiber direction. The mechanical trend was found to be better described by the lysine consumption data than by the shrinkage temperature. The biaxial loading of incrementally crosslinked collagen gels was simulated computationally with a previously published network model. Crosslinking was represented by increased fibril stiffness or by increased resistance to fibril rotation. Only the latter produced mechanical trends similar to that observed experimentally. Representing crosslinking as increased fibril stiffness did not reproduce the decreased coupling between the fiber and cross-fiber directions. The study concludes that the mechanical changes in crosslinked collagen gels are caused by the microstructural mechanism of increased resistance to fibril rotation.
Conflict of interest statement
DECLARATION OF INTEREST
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Figures
Fig.1
(a) Gelation of collagen solution in mold. The mold consisted of a round upper polypropylene piece attached to a square lower base with vacuum grease. Removable needles were placed within the mold to leave holes in the gel after solidification. The mold setup was placed within a petri-dish. Strain markers were embedded in the gel before it solidified completely. (b) Anisotropic gel following two-days of cell-compaction. The upper piece of the mold was removed after the gel solidification stage in (a) to allow free media exchange. Two sets of needles on opposite sides were removed to present anisotropic constraints for cell compaction. The gel was incubated for 2 days, during which it was compacted in an anisotropic manner (c) Anisotropic crosslinked gel attached to biaxial loading device for testing. The compacted gels from (b) were exposed to the crosslinking solution and prepared for mechanical testing. Loops of silk thread were sutured along with polypropylene floats into the needle holes in the gel. The horizontal direction is the direction of predominant fiber orientation and is referred to the ‘fiber direction’. The vertical direction is referred to as the ‘cross-fiber’ direction.
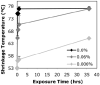
Fig.2. Screening of crosslinking conditions
Shrinkage temperature vs. exposure time for collagen gels crosslinked at different glutaraldehyde concentrations. Shrinkage temperature is a measure of the extent of crosslinking.
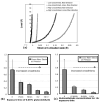
Fig.3. Glutaraldehyde crosslinking and cross-fiber strain to preload
(a) Equi-biaxial loading to 15g for case of low-crosslinked and high-crosslinked gel, with strains referenced to unloaded state of gel. The conditions for low and high crosslinking are 1hr exposure to 0.006% glutaraldehyde, and 36 hours exposure to 0.06% glutaraldehyde, respectively. The high-crosslinked gel (black) shows decreased cross-fiber strains (EP22) to the low force preload of 0.2gm, compared to the low-crosslinked one (grey). (b,c) Trends in the cross-fiber preload strain with increased crosslinking. Crosslinking was increased by either increasing the exposure time at constant glutaraldehyde concentration of 0.06% w/v (b) or by increasing the glutaraldehyde concentration at constant exposure time of 1 hour (c). In both cases, cross-fiber strains to preload decreased progressively with increasing crosslinking. The trend suggests that crosslinking decreased the cross-fiber gel compliance at low forces.
Fig.4. Glutaraldehyde crosslinking and cross-fiber strain
(a) Equi-biaxial loading to 15g shown for low-crosslinked and high-crosslinked gels, with the strains referenced to preloaded state (Ep). The conditions for low and high crosslinking are 1hr exposure to 0.006% glutaraldehyde, and 36 hours exposure to 0.06% glutaraldehyde, respectively. The cross-fiber strain (EP22) at 15 gm is greater for the high crosslinked gel. (b,c) Trends in cross-fiber strain (EP22) with increased crosslinking. Crosslinking was increased by increasing exposure time at a constant glutaraldehyde concentration of 0.06% w/v (b), or by increasing glutaraldehyde concentration at a constant exposure time of 1 hour (c). While the trends suggest that the cross-fiber strain increases with crosslinking, they are not statistically significant (see text).
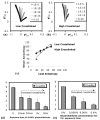
Fig.5. Glutaraldehyde crosslinking and strain-load coupling
(a,b) Biaxial strains (EP22 vs EP11) for the different loading protocols (legend) shown for the case of low crosslinked gel (a) and high crosslinked gel (b). The spread of the biaxial curves, which indicates the coupling between the biaxial strain and load anisotropy, is smaller for the high crosslink case. (c) The coupling was quantified as the slope of the biaxial strain vs. biaxial load anisotropy. (d,e) The coupling between the strain and load anisotropy decreased progressively with increased crosslinking.
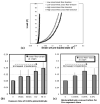
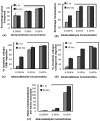
Fig.6. Biochemical analysis of collagen gels crosslinked with glutaraldehyde concentrations of 0.006%, 0.06%, and 0.6%, for exposure times of 1 and 36 hours
(a) Lysine consumption, (b) Gel shrinkage temperature, (c) moles of glutaraldehyde per mole of lysine consumed, (d) % resistance to CNBr digestion, (e) % resistance to collagenase digestion. The lysine consumption, a measure of the number of crosslinked lysines, increased with glutaraldehyde concentration only. The shrinkage temperature, a measure of collagen crosslinks and glutaraldehyde polymerization, increased with both glutaraldehyde concentration and exposure time. The biochemical data in (c),(d), and (e) showed trends similar to the shrinkage temperature.
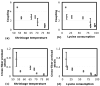
Fig.7. Relation between mechanical and biochemical trends
(a,b) Average strain-load coupling plotted against shrinkage temperature (a) and lysine consumption (b) for different crosslinking conditions. The average coupling generally decreases with both, and no localized deviations from the trend are visible due to the large standard deviations. (c,d) The average cross-fiber strain to preload (EP22) plotted against shrinkage temperature (c) and lysine consumption (d) for different crosslinking conditions. In the plot against shrinkage temperature (c), there is a significant deviation from the general trends between 56°C and 65°C. The relation with lysine consumption is more consistent (d).
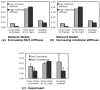
Fig.8. Model predictions for effects of two microstructural mechanisms on cross-fiber strain
Increasing fibril stiffness (a) reduced cross-fiber strain at 15g relative to preload and slightly increased coupling. Increasing rotational stiffness (b) reduced cross-fiber strain at preload, slightly increased cross-fiber strain at 15g relative to preload, and decreased coupling. Experimental data (c) for 0.06% glutaraldehyde treatment for 1 hour (low crosslinking) and 36 hours (high crosslinking) showed the same trends as increasing rotational stiffness in the model but did not match model results for increased axial stiffness.
Similar articles
- Biaxial mechanical/structural effects of equibiaxial strain during crosslinking of bovine pericardial xenograft materials.
Langdon SE, Chernecky R, Pereira CA, Abdulla D, Lee JM. Langdon SE, et al. Biomaterials. 1999 Jan;20(2):137-53. doi: 10.1016/s0142-9612(98)00142-2. Biomaterials. 1999. PMID: 10022783 - The chemical protecting group concept applied in crosslinking of natural tissues with glutaraldehyde acetals.
Goissis G, Yoshioka SA, Braile DM, Ramirez VD. Goissis G, et al. Artif Organs. 1998 Mar;22(3):210-4. doi: 10.1046/j.1525-1594.1998.06006.x. Artif Organs. 1998. PMID: 9527281 - A fibril-based structural constitutive theory reveals the dominant role of network characteristics on the mechanical behavior of fibroblast-compacted collagen gels.
Feng Z, Ishiguro Y, Fujita K, Kosawada T, Nakamura T, Sato D, Kitajima T, Umezu M. Feng Z, et al. Biomaterials. 2015 Oct;67:365-81. doi: 10.1016/j.biomaterials.2015.07.038. Epub 2015 Jul 21. Biomaterials. 2015. PMID: 26247391 - Chemical crosslinking of biopolymeric scaffolds: Current knowledge and future directions of crosslinked engineered bone scaffolds.
Oryan A, Kamali A, Moshiri A, Baharvand H, Daemi H. Oryan A, et al. Int J Biol Macromol. 2018 Feb;107(Pt A):678-688. doi: 10.1016/j.ijbiomac.2017.08.184. Epub 2017 Sep 14. Int J Biol Macromol. 2018. PMID: 28919526 Review. - In the beginning there were soft collagen-cell gels: towards better 3D connective tissue models?
Brown RA. Brown RA. Exp Cell Res. 2013 Oct 1;319(16):2460-9. doi: 10.1016/j.yexcr.2013.07.001. Epub 2013 Jul 12. Exp Cell Res. 2013. PMID: 23856376 Review.
Cited by
- Optimization of Collagen Chemical Crosslinking to Restore Biocompatibility of Tissue-Engineered Scaffolds.
Islam MM, AbuSamra DB, Chivu A, Argüeso P, Dohlman CH, Patra HK, Chodosh J, González-Andrades M. Islam MM, et al. Pharmaceutics. 2021 Jun 3;13(6):832. doi: 10.3390/pharmaceutics13060832. Pharmaceutics. 2021. PMID: 34204956 Free PMC article. - Adventitial Collagen Cross-Linking by Glutaraldehyde Reinforcing Human Saphenous Vein - Implication for Coronary Artery Bypass Grafting.
Liu C, Chen D, Li Z, Xu H, Gu C. Liu C, et al. Braz J Cardiovasc Surg. 2022 Aug 16;37(4):439-446. doi: 10.21470/1678-9741-2020-0587. Braz J Cardiovasc Surg. 2022. PMID: 35976203 Free PMC article. - Nature-Based Biomaterials and Their Application in Biomedicine.
Troy E, Tilbury MA, Power AM, Wall JG. Troy E, et al. Polymers (Basel). 2021 Sep 28;13(19):3321. doi: 10.3390/polym13193321. Polymers (Basel). 2021. PMID: 34641137 Free PMC article. Review. - Inelastic behaviour of collagen networks in cell-matrix interactions and mechanosensation.
Mohammadi H, Arora PD, Simmons CA, Janmey PA, McCulloch CA. Mohammadi H, et al. J R Soc Interface. 2015 Jan 6;12(102):20141074. doi: 10.1098/rsif.2014.1074. J R Soc Interface. 2015. PMID: 25392399 Free PMC article. - Experimental Investigation on the Penetrability Mechanism of Gel Slug During Well Completion Processes.
Dou X, Du Y, Zhang Y, Qian K. Dou X, et al. ACS Omega. 2023 Jun 15;8(25):23112-23119. doi: 10.1021/acsomega.3c02494. eCollection 2023 Jun 27. ACS Omega. 2023. PMID: 37396226 Free PMC article.
References
- Brinckmann J, Notbohm H, Müller PK, editors. Collagen - primer in structure, processing and assembly. Springer; 2005.
- Stenzel KH, Miyata T, Rubin AL. Collagen as a biomaterial. Annual Review of Biophysics and Bioengineering. 1974;3(1):231–53. - PubMed
- Eyre DR, Paz MA, Gallop PM. Cross-Linking in Collagen and Elastin. Annual Review of Biochemistry. 1984;53(1):717–48. - PubMed
- McCormick RJ, Thomas DP. Collagen crosslinking in the heart: relationship to development and function. Basic Appl Myol. 1998;8(2):143–50.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources