Inhalation exposure to smoke from synthetic "marijuana" produces potent cannabimimetic effects in mice - PubMed (original) (raw)
Inhalation exposure to smoke from synthetic "marijuana" produces potent cannabimimetic effects in mice
Jason M Wiebelhaus et al. Drug Alcohol Depend. 2012.
Abstract
Background: Use of synthetic "marijuana" has increased in recent years, produced adverse effects and prompted the temporary DEA ban of five specific cannabinoid analogs, including JWH-018. The objectives of the current study include determining the chemical content of the herbal product, Buzz, assessing its behavioral effects upon inhalation exposure to mice, determining whether CB(1) receptors mediate its pharmacological activity, and ascertaining its biodisposition in blood and various organs.
Methods: Using a nose-only exposure system, mice were exposed to smoke produced from combustion of an herbal incense product, Buzz, which contained 5.4% JWH-018. Cannabimimetic effects following smoke exposure were evaluated using the tetrad procedure, consisting of the following indices: hypomotility, antinociception, catalepsy, and hypothermia. Additionally, blood and tissues were collected for JWH-018 quantification.
Results: Inhalation exposure to Buzz produced dose-related tetrad effects similar to marijuana as well as dose-related increased levels of JWH-018 in the blood, brain, heart, kidney, liver, lung, and spleen. The behavioral effects were blocked by rimonabant, a CB(1) receptor antagonist. Effects produced by Buzz were similar in magnitude and time-course to those produced by marijuana, though equipotent doses of Buzz and marijuana yielded considerably lower brain levels of JWH-018 than THC for the respective materials.
Conclusions: Inhalation exposure to a product containing JWH-018 penetrates into the brain and other organs and produces CB(1) receptor-mediated behavioral pharmacological effects in mice. The increased potency of JWH-018 compared to THC, the variable amount of drug added to various herbal products, and unknown toxicity, undoubtedly contribute to public health risks of synthetic cannabinoids.
Copyright © 2012 Elsevier Ireland Ltd. All rights reserved.
Figures
Figure 1
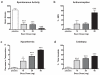
Buzz dose-dependently produces cannabinoid behavioral tetrad responses including (a) decreases in spontaneous locomotor activity, (b) antinociception in the radiant heat tail-flick test, (c) hypothermia, and (d) catalepsy in the ring immobility test. Mice were exposed via a nose-only exposure system to smoke produced from the incineration of placebo (50 mg), 10, 20, or 50 mg Buzz plant material. Baseline body temperature and tail flick latencies are found in Table 2.*p<0.05; ***p<0.001 vs. mice treated with 50 mg placebo smoke; Dunnett’s post-hoc test. All data reflect mean ± SEM; n=6 mice/group.
Figure 2
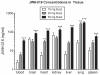
JWH-018 concentrations collected from blood, brain, heart, kidney, liver, lung, and spleen 1 h after mice were exposed to smoke produced from incineration of Buzz (10, 20, or 50 mg). JWH-018 was not detected in blood or tissue of mice exposed to placebo (not shown). **p<0.01; ***p<0.001 vs. mice treated with 50 mg placebo smoke; Dunnett’s post-hoc test. All data reflect mean ± SEM; n=4-6 mice/group of JWH-018 concentrations (ng/ml).
Figure 3
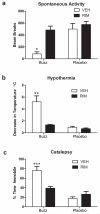
Pharmacological effects produced by inhalation of smoke from incinerated Buzz (20 mg) are CB1 receptor mediated. Rimonabant (3 mg/kg) significantly blocked Buzz-induced (a) decreases in spontaneous locomotor activity, (b) hypothermia, and (c) catalepsy in the ring immobility test. Baseline body temperatures are found in Table 2. *p<0.05; **p<0.01; ***p<0.001. Bonferroni’s multiple comparisons post-hoc test. All data reflect mean ± SEM; n=6 mice/group.
Figure 4
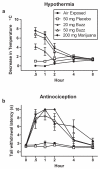
Incinerated Buzz and marijuana smoke dose-dependently produced cannabinoid behavioral responses including (a) hypothermia and (b) antinociception in the hot water bath tail withdrawal test. Mice were tested before exposure (i.e. 0 h or pre-exposure) and 30, 60, 120, 240 and 480 min after nose-only exposure to air, placebo, Buzz (20 or 50 mg) and marijuana (200 mg). Pre-exposure mean (± SEM) baseline temperatures were 38.20±0.15, 38.62±0.14, 38.55±0.10, 38.60±0.10, and 38.57± 0.15 °C for mice exposed to air, 50 mg placebo, 20 mg Buzz, 50 mg Buzz, and 200 mg marijuana, respectively. After 480 min (i.e. 8 h) mean (± SEM) body temperatures were 36.97±0.17, 37.25±0.28, 37.18 ±0.11, 37.07±0.15, and 37.05±0.18 °C for mice exposed to air, 50 mg placebo, 20 mg Buzz, 50 mg Buzz, and 200 mg marijuana, respectively. Baseline tail withdrawal latencies are found in Table 2. All data reflect mean ± SEM; n=6 mice/group.
Similar articles
- Determination of naphthalen-1-yl-(1-pentylindol-3-yl)methanone (JWH-018) in mouse blood and tissue after inhalation exposure to 'buzz' smoke by HPLC/MS/MS.
Poklis JL, Amira D, Wise LE, Wiebelhaus JM, Haggerty BJ, Lichtman AH, Poklis A. Poklis JL, et al. Biomed Chromatogr. 2012 Nov;26(11):1393-8. doi: 10.1002/bmc.2710. Epub 2012 Mar 8. Biomed Chromatogr. 2012. PMID: 22407432 Free PMC article. - Monohydroxylated metabolites of the K2 synthetic cannabinoid JWH-073 retain intermediate to high cannabinoid 1 receptor (CB1R) affinity and exhibit neutral antagonist to partial agonist activity.
Brents LK, Gallus-Zawada A, Radominska-Pandya A, Vasiljevik T, Prisinzano TE, Fantegrossi WE, Moran JH, Prather PL. Brents LK, et al. Biochem Pharmacol. 2012 Apr 1;83(7):952-61. doi: 10.1016/j.bcp.2012.01.004. Epub 2012 Jan 18. Biochem Pharmacol. 2012. PMID: 22266354 Free PMC article. - Delta9-tetrahydrocannbinol accounts for the antinociceptive, hypothermic, and cataleptic effects of marijuana in mice.
Varvel SA, Bridgen DT, Tao Q, Thomas BF, Martin BR, Lichtman AH. Varvel SA, et al. J Pharmacol Exp Ther. 2005 Jul;314(1):329-37. doi: 10.1124/jpet.104.080739. Epub 2005 Apr 14. J Pharmacol Exp Ther. 2005. PMID: 15831444 - Moving around the molecule: relationship between chemical structure and in vivo activity of synthetic cannabinoids.
Wiley JL, Marusich JA, Huffman JW. Wiley JL, et al. Life Sci. 2014 Feb 27;97(1):55-63. doi: 10.1016/j.lfs.2013.09.011. Epub 2013 Sep 23. Life Sci. 2014. PMID: 24071522 Free PMC article. Review. - Combination Chemistry: Structure-Activity Relationships of Novel Psychoactive Cannabinoids.
Wiley JL, Marusich JA, Thomas BF. Wiley JL, et al. Curr Top Behav Neurosci. 2017;32:231-248. doi: 10.1007/7854_2016_17. Curr Top Behav Neurosci. 2017. PMID: 27753007 Review.
Cited by
- Inhaled delivery of Δ(9)-tetrahydrocannabinol (THC) to rats by e-cigarette vapor technology.
Nguyen JD, Aarde SM, Vandewater SA, Grant Y, Stouffer DG, Parsons LH, Cole M, Taffe MA. Nguyen JD, et al. Neuropharmacology. 2016 Oct;109:112-120. doi: 10.1016/j.neuropharm.2016.05.021. Epub 2016 May 30. Neuropharmacology. 2016. PMID: 27256501 Free PMC article. - Δ9-tetrahydrocannabinol discrimination: Effects of route of administration in mice.
Marusich JA, Wiley JL. Marusich JA, et al. Drug Alcohol Depend Rep. 2023 Nov 10;9:100205. doi: 10.1016/j.dadr.2023.100205. eCollection 2023 Dec. Drug Alcohol Depend Rep. 2023. PMID: 38045495 Free PMC article. - Intracerebral Hemorrhage with Multiple Intracranial Arterial Stenoses in a Synthetic Cannabinoid "Spice" User.
Aydin S, Yuksel O, Aydin AE, Kizilkilic O, Celik SE. Aydin S, et al. Asian J Neurosurg. 2018 Apr-Jun;13(2):522-524. doi: 10.4103/ajns.AJNS_48_16. Asian J Neurosurg. 2018. PMID: 29682077 Free PMC article. - MAM-2201, One of the Most Potent-Naphthoyl Indole Derivative-Synthetic Cannabinoids, Exerts Toxic Effects on Human Cell-Based Models of Neurons and Astrocytes.
Coccini T, De Simone U, Lonati D, Scaravaggi G, Marti M, Locatelli CA. Coccini T, et al. Neurotox Res. 2021 Aug;39(4):1251-1273. doi: 10.1007/s12640-021-00369-3. Epub 2021 May 4. Neurotox Res. 2021. PMID: 33945101 - In Vivo Bio-Activation of JWH-175 to JWH-018: Pharmacodynamic and Pharmacokinetic Studies in Mice.
Tirri M, Arfè R, Bilel S, Corli G, Marchetti B, Fantinati A, Vincenzi F, De-Giorgio F, Camuto C, Mazzarino M, Barbieri M, Gaudio RM, Varani K, Borea PA, Botrè F, Marti M. Tirri M, et al. Int J Mol Sci. 2022 Jul 21;23(14):8030. doi: 10.3390/ijms23148030. Int J Mol Sci. 2022. PMID: 35887377 Free PMC article.
References
- Aceto MD, Scates SM, Razdan RK, Martin BR. Anandamide, an endogenous cannabinoid, has a very low physical dependence potential. J. Pharmacol. Exp. Ther. 1998;287:598–605. - PubMed
- Auwarter V, Dresen S, Weinmann W, Muller M, Putz M, Ferreiros N. ‘Spice’ and other herbal blends: harmless incense or cannabinoid designer drugs? JMS. 2009;44:832–837. - PubMed
- Coulter C, Garnier M, Moore C. Synthetic cannabinoids in oral fluid. J. Anal. Toxicol. 2011;35:424–430. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P50 DA005274/DA/NIDA NIH HHS/United States
- R01DA03672/DA/NIDA NIH HHS/United States
- R01 DA003672/DA/NIDA NIH HHS/United States
- 5P01DA009789/DA/NIDA NIH HHS/United States
- P50DA005274/DA/NIDA NIH HHS/United States
- 1F31DA030869/DA/NIDA NIH HHS/United States
- P01 DA009789/DA/NIDA NIH HHS/United States
- F31 DA030869/DA/NIDA NIH HHS/United States
LinkOut - more resources
Full Text Sources