A comparison of inflammatory and oxidative stress markers in adipose tissue from weight-matched obese male and female mice - PubMed (original) (raw)
Comparative Study
A comparison of inflammatory and oxidative stress markers in adipose tissue from weight-matched obese male and female mice
Karen J Nickelson et al. Exp Diabetes Res. 2012.
Abstract
Expansion of intra-abdominal adipose tissue and the accompanying inflammatory response has been put forward as a unifying link between obesity and the development of chronic diseases. However, an apparent sexual dimorphism exists between obesity and chronic disease risk due to differences in the distribution and abundance of adipose tissue. A range of experimental protocols have been employed to demonstrate the role of estrogen in regulating health benefits; however, most studies are confounded by significant differences in body weight and adiposity. Therefore, the purpose of this study was to compare weight-matched obese male and female mice to determine if the sex-dependent health benefits remain when body weight is similar. The development of obesity in female mice receiving a high-fat diet was delayed; however, subsequent comparisons of weight-matched obese mice revealed greater adiposity in obese female mice. Despite excess adiposity and enlarged adipocyte size, obese females remained more glucose tolerant than weight-matched male mice, and this benefit was associated with increased expression of adiponectin and reductions in immune cell infiltration and oxidative stress in adipose tissue. Therefore, the protective benefits of estrogen persist in the obese state and appear to improve the metabolic phenotype of adipose tissue and the individual.
Figures
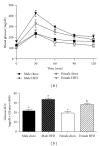
Figure 1
Obese female mice (female HFD) have improved glucose tolerance when compared to weight-matched obese male mice (male HFD). Male and female C57BL/6 mice were fed either a standard rodent chow or a high-fat diet (HFD) from 6 weeks of age until the HFD-fed group achieved a body weight of 45 g. At that time, a glucose tolerance test was performed in both chow- and HFD-fed males (21 weeks old) or females (38 weeks old) and blood glucose change over time plotted (a). Corresponding blood glucose area under the curve (AUC) was calculated (b), data are reported as mean ± SE and means with different superscripts differ by P < 0.05. n = 5–8 per group.
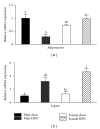
Figure 2
Adiponectin mRNA expression in gonadal adipose tissue is reduced by obesity in male mice but not weight-matched obese female mice. Male and female C57BL/6 mice were fed either a standard rodent chow or a high-fat diet (HFD) from 6 weeks of age until the HFD-fed group achieved a body weight of 45 g. At that time, both chow- and HFD-fed males (21 weeks old) or females (38 weeks old) were sacrificed and qRT PCR performed on gonadal adipose tissue. Relative mRNA expression of adiponectin was reduced by obesity in male mice, while obesity had no effect on adiponectin expression in female mice (a). Consistent with an obese phenotype, mRNA expression of leptin was elevated in both male HFD and female HFD mice (b). Data are reported as mean ± SE; n = 5–8 per group; means with different superscripts differ by P < 0.05.
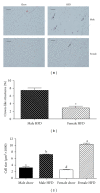
Figure 3
Obese female mice have larger adipocytes and reduced prevalence of crown-like structures in gonadal adipose tissue when compared to weight-matched obese male mice. Male and female C57BL/6 mice were fed either a standard rodent chow or a high-fat diet (HFD) from 6 weeks of age until the HFD-fed group achieved a body weight of 45 g. At that time, both chow- and HFD-fed males (21 weeks old) or females (38 weeks old) were sacrificed and histological analysis was performed on gonadal adipose tissue. Representative H&E stains of gonadal adipose tissue from each of the four treatment groups are presented in panel (a). Sections were used to quantify the presence of crown-like structure (b) and to calculate average adipocyte area (c). Data are reported as mean ± SE; n = 5–8 per group; means with different superscripts differ by P < 0.05; *P < 0.05; bar = 100 _μ_M.
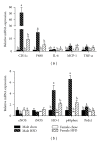
Figure 4
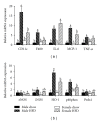
Relative mRNA expression of markers for immune cell infiltration and oxidative stress is decreased in gonadal adipose tissue isolated from obese female mice as compared to obese male mice. Male and female C57BL/6 mice were fed either a standard rodent chow or a high-fat diet (HFD) from 6 weeks of age until the HFD-fed group achieved a body weight of 45 g. At that time, both chow- and HFD-fed males (21 weeks old) or females (38 weeks old) were sacrificed and qRT PCR was performed on gonadal adipose tissue. Relative mRNA expression of markers for immune cell infiltration and inflammation (a) as well as oxidative stress (b) was determined. Data are reported as mean ± SE; n = 5–8 per group; means with different superscripts differ by P < 0.05. IL-6: interleukin-6; MCP-1: monocyte chemoattractant protein-1; TNF-α: tumor necrosis factor-alpha; eNOS: endothelial nitric oxide synthase; iNOS: inducible nitric oxide synthase; HO-1: heme oxygenase-1; p40phox: NADPH subunit p40phox; Prdx1: peroxiredoxin-1.
Figure 5
Relative mRNA expression of markers for immune cell infiltration and oxidative stress is altered in subcutaneous adipose tissue isolated from obese female mice as compared to obese male mice. Male and female C57BL/6 mice were fed either a standard rodent chow or a high-fat diet (HFD) from 6 weeks of age until the HFD-fed group achieved a body weight of 45 g. At that time, both chow- and HFD-fed males (21 weeks old) or females (38 weeks old) were sacrificed and qRT PCR was performed on subcutaneous adipose tissue. Relative mRNA expression of markers for immune cell infiltration and inflammation (a) as well as oxidative stress (b) was determined. Data are reported as mean ± SE; n = 5–8 per group; means with different superscripts differ by P < 0.05. IL-6: interleukin-6; MCP-1: monocyte chemoattractant protein-1; TNF-α: tumor necrosis factor-alpha; eNOS: endothelial nitric oxide synthase; iNOS: inducible nitric oxide synthase; HO-1: heme oxygenase-1; p40phox: NADPH subunit p40phox; Prdx1: peroxiredoxin-1.
Similar articles
- Aging exacerbates obesity-induced oxidative stress and inflammation in perivascular adipose tissue in mice: a paracrine mechanism contributing to vascular redox dysregulation and inflammation.
Bailey-Downs LC, Tucsek Z, Toth P, Sosnowska D, Gautam T, Sonntag WE, Csiszar A, Ungvari Z. Bailey-Downs LC, et al. J Gerontol A Biol Sci Med Sci. 2013 Jul;68(7):780-92. doi: 10.1093/gerona/gls238. Epub 2012 Dec 3. J Gerontol A Biol Sci Med Sci. 2013. PMID: 23213032 Free PMC article. - Antioxidant-upregulated mesenchymal stem cells reduce inflammation and improve fatty liver disease in diet-induced obesity.
Domingues CC, Kundu N, Kropotova Y, Ahmadi N, Sen S. Domingues CC, et al. Stem Cell Res Ther. 2019 Sep 2;10(1):280. doi: 10.1186/s13287-019-1393-8. Stem Cell Res Ther. 2019. PMID: 31477174 Free PMC article. - A microarray analysis of sexual dimorphism of adipose tissues in high-fat-diet-induced obese mice.
Grove KL, Fried SK, Greenberg AS, Xiao XQ, Clegg DJ. Grove KL, et al. Int J Obes (Lond). 2010 Jun;34(6):989-1000. doi: 10.1038/ijo.2010.12. Epub 2010 Feb 16. Int J Obes (Lond). 2010. PMID: 20157318 Free PMC article. - Estrogen regulates sex-specific localization of regulatory T cells in adipose tissue of obese female mice.
Ishikawa A, Wada T, Nishimura S, Ito T, Okekawa A, Onogi Y, Watanabe E, Sameshima A, Tanaka T, Tsuneki H, Saito S, Sasaoka T. Ishikawa A, et al. PLoS One. 2020 Apr 2;15(4):e0230885. doi: 10.1371/journal.pone.0230885. eCollection 2020. PLoS One. 2020. PMID: 32240221 Free PMC article. - Oxidative Stress Linking Obesity and Cancer: Is Obesity a 'Radical Trigger' to Cancer?
Jovanović M, Kovačević S, Brkljačić J, Djordjevic A. Jovanović M, et al. Int J Mol Sci. 2023 May 8;24(9):8452. doi: 10.3390/ijms24098452. Int J Mol Sci. 2023. PMID: 37176160 Free PMC article. Review.
Cited by
- Prolonged Consumption of A2 β-Casein Milk Reduces Symptoms Compared to A1 and A2 β-Casein Milk in Lactose Maldigesters: A Two-Week Adaptation Study.
Ramakrishnan M, Mysore Saiprasad S, Savaiano DA. Ramakrishnan M, et al. Nutrients. 2024 Jun 20;16(12):1963. doi: 10.3390/nu16121963. Nutrients. 2024. PMID: 38931316 Free PMC article. Clinical Trial. - Sexually dimorphic myeloid inflammatory and metabolic responses to diet-induced obesity.
Griffin C, Lanzetta N, Eter L, Singer K. Griffin C, et al. Am J Physiol Regul Integr Comp Physiol. 2016 Aug 1;311(2):R211-6. doi: 10.1152/ajpregu.00136.2016. Epub 2016 Jun 1. Am J Physiol Regul Integr Comp Physiol. 2016. PMID: 27252473 Free PMC article. Review. - Increased 4E-BP1 Expression Protects against Diet-Induced Obesity and Insulin Resistance in Male Mice.
Tsai SY, Rodriguez AA, Dastidar SG, Del Greco E, Carr KL, Sitzmann JM, Academia EC, Viray CM, Martinez LL, Kaplowitz BS, Ashe TD, La Spada AR, Kennedy BK. Tsai SY, et al. Cell Rep. 2016 Aug 16;16(7):1903-14. doi: 10.1016/j.celrep.2016.07.029. Epub 2016 Aug 4. Cell Rep. 2016. PMID: 27498874 Free PMC article. - Biochemical alterations during the obese-aging process in female and male monosodium glutamate (MSG)-treated mice.
Hernández-Bautista RJ, Alarcón-Aguilar FJ, Del C Escobar-Villanueva M, Almanza-Pérez JC, Merino-Aguilar H, Fainstein MK, López-Diazguerrero NE. Hernández-Bautista RJ, et al. Int J Mol Sci. 2014 Jun 27;15(7):11473-94. doi: 10.3390/ijms150711473. Int J Mol Sci. 2014. PMID: 24979131 Free PMC article. - Pyrroloquinoline-quinone to reduce fat accumulation and ameliorate obesity progression.
Mohamad Ishak NS, Ikemoto K. Mohamad Ishak NS, et al. Front Mol Biosci. 2023 May 5;10:1200025. doi: 10.3389/fmolb.2023.1200025. eCollection 2023. Front Mol Biosci. 2023. PMID: 37214340 Free PMC article. Review.
References
- Abbasi F, Brown BW, Jr., Lamendola C, McLaughlin T, Reaven GM. Relationship between obesity, insulin resistance, and coronary heart disease risk. Journal of the American College of Cardiology. 2002;40(5):937–943. - PubMed
- Flegal KM, Graubard BI, Williamson DF, Gail MH. Cause-specific excess deaths associated with underweight, overweight, and obesity. Journal of the American Medical Association. 2007;298(17):2028–2037. - PubMed
- Ferrante AW., Jr. Obesity-induced inflammation: a metabolic dialogue in the language of inflammation. Journal of Internal Medicine. 2007;262(4):408–414. - PubMed
- Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444(7121):860–867. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical