Amyloid β-protein aggregation produces highly reproducible kinetic data and occurs by a two-phase process - PubMed (original) (raw)
Amyloid β-protein aggregation produces highly reproducible kinetic data and occurs by a two-phase process
Erik Hellstrand et al. ACS Chem Neurosci. 2010.
Abstract
Protein aggregation can lead to major disturbances of cellular processes and is associated with several diseases. We report kinetic and equilibrium data by ThT fluorescence and enzyme-linked immunosorbent assay of sufficient quality and reproducibility to form a basis for mechanistic understanding of amyloid β-peptide (Aβ) fibril formation. Starting from monomeric peptide in a pure buffer system without cosolvents, we find that the kinetics of Aβ aggregation vary strongly with peptide concentration in a highly predictable manner. The free Aβ concentration in equilibrium with fibrils was found to vary with total peptide concentration in a manner expected for a two-phase system. The free versus total Aβ concentration was linear up to ca. 0.2 μM, after which free Aβ decreased with total Aβ toward an asymptotic value. Our results imply that Aβ fibril formation arises from a sequence of events in a highly predictable manner.
Keywords: Alzheimer; Amyloid; aggregation; fibril; kinetics; mechanism.
Figures
Figure 1
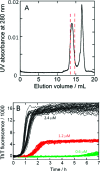
(A) Isolation of monomeric Aβ(M1−42) by gel filtration on a Superdex 75 column in 20 mM sodium phosphate buffer, pH 8, with 200 μM EDTA and 0.02% NaN3. The monomer is collected between the dashed red lines and is free from higher Aβ assembly forms (shoulder before monomer peak) and buffer and salts (tall peak after the monomer peak). (B) Kinetic traces by ThT fluorescence for 32 replicates at concentrations 2.4 (black), 1.2 (red), and 0.6 μM (green) Aβ(M1−42) in 20 mM sodium phosphate, pH 8, 200 μM EDTA, 0.02% NaN3, 20 μM ThT. The first 7 h are shown.
Figure 2
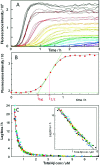
Concentration dependence of Aβ aggregation kinetics in 20 mM sodium phosphate, pH 8, 200 μM EDTA, 0.02% NaN3, 20 μM ThT. (A) Kinetics of aggregation monitored using ThT fluorescence. Data for the 13 highest concentrations in a single experiment are shown with Aβ(Μ1−42) concentrations of 5.8 (black), 4.9 (brown), 3.9 (red), 2.9 (orange), 2.6 (yellow), 2.2 (green-yellow), 1.85 (yellow-green), 1.65 (green), 1.46 (cyan), 1.31 (light blue), 1.17 (blue), 1.07 (marine blue), and 0.97 (purple) μM. (B) Fitting of eq 1 to one of the fibrillation traces in panel A, with data points as filled circles and the fitted curve as a solid line. The values for _t_1/2 as obtained by the fit and _t_lag by eq 2 are indicated. (C) Lagtime obtained by fitting eq 1 to 672 kinetic traces in seven sets of data (in black, red, green, magenta, cyan, yellow, and blue) versus Aβ(Μ1−42) concentration. Each point is average of 3−32 replicates of the same solution. The solid line is a power function with exponent −1.48 fitted to all data points. Inset: same data with logarithmic axes.
Figure 3
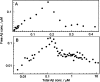
Concentration dependence of Aβ aggregation equilibrium in 20 mM sodium phosphate, pH 8, 200 μM EDTA, 0.02% NaN3. Samples were allowed to aggregate for 84−96 h, and large aggregates were removed by centrifugation. The concentration of soluble Aβ determined by ELISA is plotted versus total concentration (from acid hydrolysis): (A) low concentration samples, linear axes; (B) all samples, logarithmic axes.
Similar articles
- The Japanese mutant Aβ (ΔE22-Aβ(1-39)) forms fibrils instantaneously, with low-thioflavin T fluorescence: seeding of wild-type Aβ(1-40) into atypical fibrils by ΔE22-Aβ(1-39).
Cloe AL, Orgel JP, Sachleben JR, Tycko R, Meredith SC. Cloe AL, et al. Biochemistry. 2011 Mar 29;50(12):2026-39. doi: 10.1021/bi1016217. Epub 2011 Feb 24. Biochemistry. 2011. PMID: 21291268 Free PMC article. - Steady-state and time-resolved Thioflavin-T fluorescence can report on morphological differences in amyloid fibrils formed by Aβ(1-40) and Aβ(1-42).
Lindberg DJ, Wranne MS, Gilbert Gatty M, Westerlund F, Esbjörner EK. Lindberg DJ, et al. Biochem Biophys Res Commun. 2015 Mar 6;458(2):418-23. doi: 10.1016/j.bbrc.2015.01.132. Epub 2015 Feb 7. Biochem Biophys Res Commun. 2015. PMID: 25660454 - Scanning cysteine mutagenesis analysis of Abeta-(1-40) amyloid fibrils.
Shivaprasad S, Wetzel R. Shivaprasad S, et al. J Biol Chem. 2006 Jan 13;281(2):993-1000. doi: 10.1074/jbc.M505091200. Epub 2005 Nov 1. J Biol Chem. 2006. PMID: 16263715 - Alzheimer's beta-amyloid: insights into fibril formation and structure from Congo red binding.
Inouye H, Kirschner DA. Inouye H, et al. Subcell Biochem. 2005;38:203-24. doi: 10.1007/0-387-23226-5_10. Subcell Biochem. 2005. PMID: 15709480 Review. - Electrochemistry of Alzheimer Disease Amyloid Beta Peptides.
Chiorcea-Paquim AM, Enache TA, Oliveira-Brett AM. Chiorcea-Paquim AM, et al. Curr Med Chem. 2018;25(33):4066-4083. doi: 10.2174/0929867325666180214112536. Curr Med Chem. 2018. PMID: 29446720 Review.
Cited by
- Plasmonic Nanoparticles as Optical Sensing Probes for the Detection of Alzheimer's Disease.
Oyarzún MP, Tapia-Arellano A, Cabrera P, Jara-Guajardo P, Kogan MJ. Oyarzún MP, et al. Sensors (Basel). 2021 Mar 16;21(6):2067. doi: 10.3390/s21062067. Sensors (Basel). 2021. PMID: 33809416 Free PMC article. Review. - BRICHOS domains efficiently delay fibrillation of amyloid β-peptide.
Willander H, Presto J, Askarieh G, Biverstål H, Frohm B, Knight SD, Johansson J, Linse S. Willander H, et al. J Biol Chem. 2012 Sep 7;287(37):31608-17. doi: 10.1074/jbc.M112.393157. Epub 2012 Jul 16. J Biol Chem. 2012. PMID: 22801430 Free PMC article. - Computational maturation of a single-domain antibody against Aβ42 aggregation.
Lin J, Figazzolo C, Metrick MA 2nd, Sormanni P, Vendruscolo M. Lin J, et al. Chem Sci. 2021 Oct 7;12(41):13940-13948. doi: 10.1039/d1sc03898b. eCollection 2021 Oct 27. Chem Sci. 2021. PMID: 35475123 Free PMC article. - Thermodynamic phase diagram of amyloid-β (16-22) peptide.
Wang Y, Bunce SJ, Radford SE, Wilson AJ, Auer S, Hall CK. Wang Y, et al. Proc Natl Acad Sci U S A. 2019 Feb 5;116(6):2091-2096. doi: 10.1073/pnas.1819592116. Epub 2019 Jan 23. Proc Natl Acad Sci U S A. 2019. PMID: 30674664 Free PMC article. - Ion-specific effects on prion nucleation and strain formation.
Rubin J, Khosravi H, Bruce KL, Lydon ME, Behrens SH, Chernoff YO, Bommarius AS. Rubin J, et al. J Biol Chem. 2013 Oct 18;288(42):30300-30308. doi: 10.1074/jbc.M113.467829. Epub 2013 Aug 29. J Biol Chem. 2013. PMID: 23990463 Free PMC article.
References
- Selkoe D. J. (2001) Alzheimer's disease: Genes, proteins, and therapy. Physiol. Rev. 81, 742–761. - PubMed
- Walsh D. M.; Selkoe D. J. (2007) A beta oligomers- a decade of discovery. J. Neurochem. 101, 1172–1184. - PubMed
- Walsh D. M.; Hartley D. M.; Kusumoto Y.; Fezoui Y.; Margaret M.; Condron M. M.; Lomakin A.; Benedek G. B.; Selkoe D. J.; Teplow D. B. (1999) Amyloid beta-protein fibrillogenesis. Structure and biological activity of protofibrillar intermediates. J. Biol. Chem. 274, 25945–25952. - PubMed
- Nelson R.; Eisenberg D. (2006) Structural models of amyloid-like fibrils. Adv. Protein Chem. 73, 235–282. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources