Combination delivery of TGF-β inhibitor and IL-2 by nanoscale liposomal polymeric gels enhances tumour immunotherapy - PubMed (original) (raw)
. 2012 Oct;11(10):895-905.
doi: 10.1038/nmat3355. Epub 2012 Jul 15.
Stephen H Wrzesinski, Eric Stern, Michael Look, Jason Criscione, Ragy Ragheb, Steven M Jay, Stacey L Demento, Atu Agawu, Paula Licona Limon, Anthony F Ferrandino, David Gonzalez, Ann Habermann, Richard A Flavell, Tarek M Fahmy
Affiliations
- PMID: 22797827
- PMCID: PMC3601683
- DOI: 10.1038/nmat3355
Combination delivery of TGF-β inhibitor and IL-2 by nanoscale liposomal polymeric gels enhances tumour immunotherapy
Jason Park et al. Nat Mater. 2012 Oct.
Abstract
The tumour microenvironment thwarts conventional immunotherapy through multiple immunologic mechanisms, such as the secretion of the transforming growth factor-β (TGF-β), which stunts local tumour immune responses. Therefore, high doses of interleukin-2 (IL-2), a conventional cytokine for metastatic melanoma, induces only limited responses. To overcome the immunoinhibitory nature of the tumour microenvironment, we developed nanoscale liposomal polymeric gels (nanolipogels; nLGs) of drug-complexed cyclodextrins and cytokine-encapsulating biodegradable polymers that can deliver small hydrophobic molecular inhibitors and water-soluble protein cytokines in a sustained fashion to the tumour microenvironment. nLGs releasing TGF-β inhibitor and IL-2 significantly delayed tumour growth, increased survival of tumour-bearing mice, and increased the activity of natural killer cells and of intratumoral-activated CD8(+) T-cell infiltration. We demonstrate that the efficacy of nLGs in tumour immunotherapy results from a crucial mechanism involving activation of both innate and adaptive immune responses.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
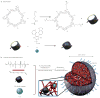
Figure 1. Fabrication of the nLG particle system
The synthesis approach consists of two steps. a, In the first step, methacrylate-f-CD was used to solubilize the TGF-β inhibitor (SB505124). NHS, N-Hydroxysuccinimide. b, In the second step, nLGs were formulated from lyophilized liposomes loaded with biodegradable crosslinking polymer, acrylated-CD-SB505 complex, and IL-2 cytokine. This core–shell structure facilitated entrapment of the drug-loaded CD (blue) and the IL-2 (green) in a biodegradable polymer matrix (red) with a PEGylated liposomal coating (grey). The degradable gel consists of central water-soluble PEG groups (n = 200 repeats), lactide groups (m ~ 2.5), and terminal acrylate groups. After loading, photoinduced polymerization of the polymer and acrylated-CD results in gel formation.
Figure 2. Controlled release clearance and biodistribution in healthy animals
a, Cumulative IL-2 and drug released from co-loaded nLGs normalized by carrier mass. Error bars in all plots represent ± 1 s.d. All experiments were repeated at least twice with similar results. b, Clearance of drug dose: encapsulation in nLGs significantly increased the remaining percentage of initial dose in the blood at 1 and 24 h post-injection (two population _t_-test, P < 0.01, ###). Mice received a single dose of rhodamine-loaded nLG or soluble rhodamine (in saline) via intravenous tail-vein injection. Animals were euthanized at 1, 24, 48 and 72 h post-injection for extraction and quantification of fluorescence. c, Whole body biodistribution: significantly higher (two population _t_-test, P < 0.01) amounts of rhodamine were detected in the major organs of nLG-treated animals (top panel) compared with animals injected with free dye (bottom panel). Data are presented as mean percentage of initial dose given and error bars represent ± 1 s.d. averaged across at least three mice per time point. d, Time-dependent accumulation in subcutaneous tumour: cumulative rhodamine tumour penetration (red) after B16 peritumoral injection in B6 mice. Peritumoral tissue was collected to quantify the remaining dose of nLG surrounding the tumour (black).
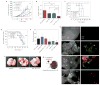
Figure 3. Clinical effects of nLG therapy on subcutaneous and metastatic melanoma
a, Plot of tumour area versus time (day 0 was the day of tumour cell injection). Red arrows indicate treatments (via intratumoral injection). Mice bearing subcutaneous tumours were euthanized either when the greatest tumour dimension was larger than 15 mm or when exhibiting signs of illness. Tumour areas of deceased mice were not included after the day of death. Each group initially contained five mice, except for the nLG–SB + IL-2 group, which contained four. Error bars represent ± 1 s.d. Tumours in the nLG–SB and nLG–SB + IL-2 groups were significantly smaller when compared against all other groups from day 12 to day 22 (P < 0.05, *, for nLG–SB, and P < 0.001, * * *, for nLG–SB-IL+2, versus no treatment, soluble SB, soluble SB + IL-2, and nLG–IL-2 groups by ANOVA with Tukey’s multiple comparison test). Tumours in the nLG–SB + IL-2 group were also significantly smaller than in the nLG–SB group from day 12 until day 26 (p < 0.05, #, by two-tailed _t_-test). b, Tumour masses of nLG-treated groups seven days after treatment. Mice were euthanized directly before tumour mass determination. Error bars represent ± 1 s.d. averaged across six (nLG–empty), ten (nLG–IL-2), nine (nLG–SB) and ten (nLG–SB + IL-2) mice. Each group initially contained ten mice. The nLG–SB + IL-2 group had significantly lower tumour masses than those of the nLG–empty (P < 0.001, * * *), nLG–IL-2 (P < 0.01, **) and nLG–SB (P < 0.01, **) groups. Tumour masses in the nLG–IL-2 and nLG–SB groups were also significantly lower than those in the nLG–empty (P < 0.05, *) group. All statistical comparisons were performed with an ANOVA using Tukey’s post-test. c, Survival plot of mice from the same study given in a. Red arrows denote treatment days. The survival of mice treated with nLG–SB was significantly longer by Mantel–Cox and Gehan–Breslow–Wilcoxon analyses (P < 0.01), and nLG–SB + IL-2 significantly extended survival by both analyses (P < 0.001). Studies were repeated 2–3 times with similar results. d, Survival plot of mice after systemic therapy. Red arrows denote treatment days. The survival of mice treated with nLG–SB + IL-2 was significantly higher by Gehan–Breslow–Wilcoxon analysis (P < 0.05). Studies were repeated 2–3 times with similar results. e, Number of tumours counted by blind observers in lungs of mice 14 days after initiation of treatment. Lung metastatic regions ranged in size from 0.5–2 mm in diameter. Treatment with nLG–SB, nLG–IL-2 and nLG–SB + IL-2 significantly reduced the number of metastases (P < 0.05, *, or P < 0.01, **, by ANOVA with Tukey’s post-test) when compared with soluble SB. Data represent mean ± 1 s.d. averaged across at least six mice per treatment group. f, Representative lung images from mice immediately before collection of lung-infiltrating lymphocytes. g, Visualization of nanopliogel trafficking to metastatic tumours. The distribution of both nLG carrier and encapsulated payload was investigated using dual-labelled NLG; fluorescein-labelled phosphoethanolamine was incorporated into the lipid component of CD-rhodamine-loaded nLGs. h, Analysis of lung tissues under bright field and fluorescent microscopy demonstrate the presence of both lipid carrier (green) and rhodamine payload (red) around individual lung tumours at 2 h post injection. Lipid fluorescence was significantly diminished four days after injection.
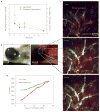
Figure 4. Biodistribution to subcutaneous tumours after systemic administration
a, Rhodamine and lipid were extracted from subcutaneous tumours following intravenous tail-vein injection at day 0. Experiments were repeated at least twice and data represent mean ± s.d. of 3–4 mice per time point. b, Whole-mount wide-field images demonstrating accumulation of both lipids and rhodamine in the peritumoral space. BF, Bright Field; FL, Fluorescence. c, Demonstration of nLG diffusion within the subcutaneous tumour by time-resolved intravital two-photon laser scanning microscopy after intravenous injection. Images presented are an extended focus projection of two optical sections at three separate time points after intravenous injection and are representative of those obtained in three separate experiments. d, Mean pixel fluorescein and rhodamine intensities of the entire imaged tissue volume represented in c.
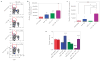
Figure 5. The adaptive immune response and mechanism of nLG–SB + IL-2 action
a, Representative FACS analyses from subcutaneous tumours receiving intratumoral injections (see Fig. 3). Panels represent, from left to right, percentage of CD4+ versus CD8+ cells in lymphocyte gate, percentage of CD8+ that are activated (CD44+CD62L−), and percentage of CD4+ that are Foxp3+ regulatory T cells. Each group contained six mice and studies were repeated 2–3 times with similar results. Data represent mean ± 1 s.d. The red circles highlight the population that is being quantitated in the charts. b, Haematoxylin (light blue) and anti-Leukocyte Common Antigen (brown) staining shows relative increases in TIL infiltration in tumours with nLG treatments. c, Absolute number of activated CD8+ cells present in lung tumours (normalized per number of tumours) for the study shown in a. All groups have significantly greater numbers (P < 0.01) compared with empty nLGs. d, Absolute number of activated CD8+ cells present in tumours (normalized per tumour mass) removed from mice seven days after treatment (same study as in Fig. 3b). Treatment with nLG–SB significantly increased activated CD8+ populations (P < 0.05), as did treatment with nLG–IL-2, or nLG–SB + IL-2 (P < 0.001), over unloaded particles (nLG–empty). Error bars represent ± 1 s.d. averaged across six (nLG–empty), ten (nLG–IL-2), nine (nLG–SB) and ten (nLG–SB + IL-2) mice. Each group initially contained ten mice. e, Activated CD8+: Treg ratio in TILs for the study shown in c. All groups have significantly greater ratios (P < 0.05) compared with empty nLGs.
Figure 6. Role of NK cells in tumour immunotherapy after combination delivery
a, Representative FACS analyses from subcutaneous tumours receiving intratumoral injections in Fig. 3. Panels represent percentage of lymphocytes that are NK cells (NK1.1+). Data represent mean ± 1 s.d. Each group contained six mice and studies were repeated 2–3 times with similar results. The red circles highlight the population that is being quantitated in the charts. b, Absolute number of NK cells present in tumours (normalized per number of tumours) for the study shown in a. Compared with the empty particle group, significantly more NKs were present in the lungs following treatment by nLG–SB + IL-2 (P < 0.05, *), nLG–SB (P < 0.05, *), and nLG–IL-2 (P < 0.01, **). c, Absolute number of NK cells present in tumours (normalized per tumour mass) for the same study as in Fig. 3b. The nLG–SB + IL-2-treated group has significantly more NKs than the control group (P < 0.01, **), the SB-treated group (P < 0.05, *), and the IL-2-treated group (P < 0.01, *). Error bars represent ± 1 s.d. averaged across six (nLG–empty), ten (nLG–IL-2), nine (nLG–SB) and ten (nLG–SB + IL-2) mice. Each group initially contained ten mice. d, Comparison of tumour masses from wild type (WT) or NK-depleted (NKD) mice euthanized seven days after initial treatment. The nLG–SB + IL-2-treated WT group has significantly smaller tumours than all other treatment groups (P < 0.001, ###). The NKD nLG–SB and nLG–SB + IL-2 groups have significantly larger tumours than their WT counterparts (both P < 0.001, * * *). Studies were repeated 2–3 times with similar results.
Comment in
- Nanoparticle immunotherapy: Combo combat.
Brinker CJ. Brinker CJ. Nat Mater. 2012 Oct;11(10):831-2. doi: 10.1038/nmat3434. Nat Mater. 2012. PMID: 23001226 No abstract available.
Similar articles
- Combined delivery of a TGF-β inhibitor and an adenoviral vector expressing interleukin-12 potentiates cancer immunotherapy.
Jiang J, Zhang Y, Peng K, Wang Q, Hong X, Li H, Fan G, Zhang Z, Gong T, Sun X. Jiang J, et al. Acta Biomater. 2017 Oct 1;61:114-123. doi: 10.1016/j.actbio.2017.05.009. Epub 2017 May 5. Acta Biomater. 2017. PMID: 28483693 - Nanoparticle immunotherapy: Combo combat.
Brinker CJ. Brinker CJ. Nat Mater. 2012 Oct;11(10):831-2. doi: 10.1038/nmat3434. Nat Mater. 2012. PMID: 23001226 No abstract available. - Human tumour immune evasion via TGF-β blocks NK cell activation but not survival allowing therapeutic restoration of anti-tumour activity.
Wilson EB, El-Jawhari JJ, Neilson AL, Hall GD, Melcher AA, Meade JL, Cook GP. Wilson EB, et al. PLoS One. 2011;6(9):e22842. doi: 10.1371/journal.pone.0022842. Epub 2011 Sep 6. PLoS One. 2011. PMID: 21909397 Free PMC article. - TGF-beta and immune cells: an important regulatory axis in the tumor microenvironment and progression.
Yang L, Pang Y, Moses HL. Yang L, et al. Trends Immunol. 2010 Jun;31(6):220-7. doi: 10.1016/j.it.2010.04.002. Epub 2010 Jun 1. Trends Immunol. 2010. PMID: 20538542 Free PMC article. Review. - Biological effects of IL-15 on immune cells and its potential for the treatment of cancer.
Zhang S, Zhao J, Bai X, Handley M, Shan F. Zhang S, et al. Int Immunopharmacol. 2021 Feb;91:107318. doi: 10.1016/j.intimp.2020.107318. Epub 2020 Dec 28. Int Immunopharmacol. 2021. PMID: 33383444 Review.
Cited by
- Enhancing cancer chemo-immunotherapy by biomimetic nanogel with tumor targeting capacity and rapid drug-releasing in tumor microenvironment.
Shang L, Jiang X, Yang T, Xu H, Xie Q, Hu M, Yang C, Kong L, Zhang Z. Shang L, et al. Acta Pharm Sin B. 2022 May;12(5):2550-2567. doi: 10.1016/j.apsb.2021.11.004. Epub 2021 Nov 9. Acta Pharm Sin B. 2022. PMID: 35646526 Free PMC article. - A nano-innate immune system activator for cancer therapy in a 4T1 tumor-bearing mouse model.
Liu XY, Zhu MH, Wang XY, Dong X, Liu HJ, Li RY, Jia SC, Lu Q, Zhao M, Sun P, Chen HZ, Fang C. Liu XY, et al. J Nanobiotechnology. 2022 Jan 29;20(1):54. doi: 10.1186/s12951-022-01265-4. J Nanobiotechnology. 2022. PMID: 35093074 Free PMC article. - Lymphatic vessels and tertiary lymphoid organs.
Ruddle NH. Ruddle NH. J Clin Invest. 2014 Mar;124(3):953-9. doi: 10.1172/JCI71611. Epub 2014 Mar 3. J Clin Invest. 2014. PMID: 24590281 Free PMC article. Review. - Poly(styrene)-b-poly(DL-lactide) copolymer-based nanoparticles for anticancer drug delivery.
Lee JY, Kim JS, Cho HJ, Kim DD. Lee JY, et al. Int J Nanomedicine. 2014 Jun 3;9:2803-13. doi: 10.2147/IJN.S62806. eCollection 2014. Int J Nanomedicine. 2014. PMID: 24940058 Free PMC article. - Increased antitumor effects using IL-2 with anti-TGF-β reveals competition between mouse NK and CD8 T cells.
Alvarez M, Bouchlaka MN, Sckisel GD, Sungur CM, Chen M, Murphy WJ. Alvarez M, et al. J Immunol. 2014 Aug 15;193(4):1709-16. doi: 10.4049/jimmunol.1400034. Epub 2014 Jul 7. J Immunol. 2014. PMID: 25000978 Free PMC article.
References
- Tawbi HA, Kirkwood JM. Management of metastatic melanoma. Semin Oncol. 2007;34:532–545. - PubMed
- Atkins MB, et al. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: Analysis of 270 patients treated between 1985 and 1993. J Clin Oncol. 1999;17:2105–2116. - PubMed
- Acquavella N, et al. Toxicity and activity of a twice daily high-dose bolus interleukin 2 regimen in patients with metastatic melanoma and metastatic renal cell cancer. J Immunother. 2008;31:569–576. - PubMed
- Gorelik L, Flavell RA. Immune-mediated eradication of tumors through the blockade of transforming growth factor-β signaling in T cells. Nature Med. 2001;7:1118–1122. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01-HL085416/HL/NHLBI NIH HHS/United States
- K99 HL112905/HL/NHLBI NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- P30 AR053495/AR/NIAMS NIH HHS/United States
- HL-55397/HL/NHLBI NIH HHS/United States
- R01 HL055397/HL/NHLBI NIH HHS/United States
- U19 AI082713/AI/NIAID NIH HHS/United States
- R01 HL085416/HL/NHLBI NIH HHS/United States
- R01-EB008260/EB/NIBIB NIH HHS/United States
- U19AI082713/AI/NIAID NIH HHS/United States
- R01 EB008260/EB/NIBIB NIH HHS/United States
- P30AR053495/AR/NIAMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials