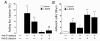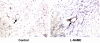Inhibition of nitric oxide synthesis enhances leukocyte rolling and adhesion in human microvasculature - PubMed (original) (raw)
Inhibition of nitric oxide synthesis enhances leukocyte rolling and adhesion in human microvasculature
Mokarram Hossain et al. J Inflamm (Lond). 2012.
Abstract
Background: Nitric oxide (NO) is a multifunctional signaling molecule that regulates important cellular events in inflammation including leukocyte recruitment. Previous studies have shown that pharmacological inhibition of NO synthesis induces leukocyte recruitment in various in vitro and animal models. However, it is not known whether NO modulation has similar effects on leukocyte-endothelial cell interactions within the human microvasculature. The present study explored the effect of systemic L-NAME treatment on leukocyte recruitment in the SCID-hu mouse model.
Methods: Human skin xenografts were transplanted in SCID mice to study human leukocyte dynamics in human vasculature. Early events of human leukocyte recruitment in human vasculature were studied using intravital microscopy. NO synthesis was pharmacologically inhibited using NG-nitro-L-arginine methyl ester (L-NAME). Immunohistochemical analysis was performed to elucidate E-selectin expression in human xenograft skin. Human neutrophil-endothelial cell interactions were also studied in an in vitro flow chamber assay system. P- and E-selectin expression on cultured human umbilical vein endothelial cells (HUVECs) was measured using ELISA. Platelet-activating factor (PAF) synthesis was detected using a TLC-based assay.
Results: L-NAME treatment significantly enhanced the rolling and adhesion of human leukocytes to the human vasculature. Functional blocking of P- and E-selectins significantly inhibited rolling but not adhesion induced by inhibition of NO synthesis. Systemic L-NAME treatment enhanced E-selectin expression in human xenograft skin. L-NAME treatment significantly enhanced P- and E-selectin expression on HUVECs. L-NAME treatment did not significantly modify neutrophil rolling or adhesion to HUVECs indicating that L-NAME-induced subtle P- and E-selectin expression was insufficient to elicit dynamic neutrophil-HUVEC interactions in vitro. Moreover, synthesis of endothelial-derived PAF was not significantly modified by L-NAME treatment. These results point to the accelerated leukocyte recruitment in human vasculature following suppression of NO synthesis, effects that are mediated by P- and E-selectins. The findings are, however, not supported by the in vitro data.
Conclusion: Inhibition of endogenous NO triggers early events of human leukocyte recruitment in human vasculature, involving complex cellular or molecular mechanisms in addition to P- and E-selectin-mediated leukocyte rolling.
Figures
Figure 1
Human leukocyte interactions with the human microvasculature. A. Representative image of a FITC-conjugated _Ulex europaeus_-stained human post-capillary venule from the control SCID-hu mouse. B. Representative image of rhodamine 6-G labelled human leukocytes rolling and adhering to a _Ulex europaeus_-stained human post-capillary venule after 4-hour L-NAME treatment (50 mg/kg b.w., i.p.) in SCID-hu mice.
Figure 2
L-NAME-elicited human leukocyte rolling and adhesion in vivo. A. Leukocyte rolling flux fraction in untreated mice (control, white bar) and L-NAME (50 mg/kg b.w., i.p.)-treated mice (black bars). ** indicates P < 0.01 difference from untreated mice (t test). # indicates P < 0.05 difference from L-NAME-treated group in the absence of functional blocking antibodies (t test). + and – indicate the presence and absence of functional blocking anti-P- and/or anti-E-selectin antibodies, respectively. Data are means ± SEM (n = 2−6; number of vessels studied = 4−13; control: n = 4; number of vessels = 7). B. Leukocyte adhesion in untreated mice (control, white bar) or L-NAME (50 mg/kg b.w., i.p.)-treated mice (black bars). * and ** indicate P < 0.05 and P < 0.01 respectively from untreated mice (t test). + and – indicate the presence and absence of functional blocking anti-P- and/or anti-E-selectin antibodies, respectively. Data are means ± SEM (n = 3−8; number of vessels studied = 4−16; control: n = 4; number of vessels = 8).
Figure 3
L-NAME-induced upregulation of E-selectin expression in vivo. Immunohistochemical analysis (representative of 4 specimens each) showing E-selectin expression (arrow) in human microvasculature of the human skin xenografts in SCID-hu mice without treatment (control, left panel) and 4 h following L-NAME administration (50 mg/kg b.w., i.p., right panel).
Figure 4
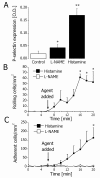
L-NAME-sensitive P-selectin expression and function in vitro. A. Endothelial P-selectin expression in controls (white bar) and after treatment with L-NAME (100 μM) or histamine (25 μM) for 10 min (black bars). * and ** indicate P < 0.05 and P < 0.01, respectively, difference from the control (t test). Data are means ± SEM (n = 4). B. Rolling neutrophils before and after treatment of HUVECs with 100 μM L-NAME (white squares) or 25 μM histamine (black squares). * indicates P < 0.05 difference from the value prior to treatment (ANOVA). Data are means ± SEM (n = 4). C. Adherent neutrophils before and after treatment of HUVECs with 100 μM L-NAME (white squares) or 25 μM histamine (black squares). * indicates P < 0.05 difference from the value prior to treatment (ANOVA). Data are means ± SEM (n = 4).
Figure 5
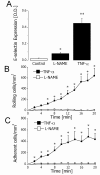
L-NAME-sensitive E-selectin expression and function in vitro. A. Endothelial E-selectin expression in controls (white bar) and 4 hours after treatment with L-NAME (100 μM) or TNFα (10 ng/mL) (black bars). * and ** indicate P < 0.05 and P < 0.01, respectively, difference from the control (t test). Data are means ± SEM (n = 4). B. Rolling neutrophils 4 h after treatment of HUVECs with 100 μM L-NAME (white squares) or 10 ng/ml TNFα (black squares). * indicates P < 0.05 difference from the value at time point 0 min (ANOVA). Data are means ± SEM (n = 4). C. Adhesion of neutrophils 4 h after treatment of HUVECs with 100 μM L-NAME (white squares) or 10 ng/mL TNFα (black squares). * indicates P < 0.05 difference from the value at time point 0 min (ANOVA). Data are means ± SEM (n = 4).
Similar articles
- Inhibition of endothelial-derived nitric oxide promotes P-selectin expression and actions in the rat microcirculation.
Davenpeck KL, Gauthier TW, Lefer AM. Davenpeck KL, et al. Gastroenterology. 1994 Oct;107(4):1050-8. doi: 10.1016/0016-5085(94)90229-1. Gastroenterology. 1994. PMID: 7523213 - Chronic blockade of nitric oxide biosynthesis in rats: effect on leukocyte endothelial interaction and on leukocyte recruitment.
Farsky SH, Borelli P, Fock RA, Proto SZ, Ferreira JM Jr, Mello SB. Farsky SH, et al. Inflamm Res. 2004 Sep;53(9):442-52. doi: 10.1007/s00011-004-1288-7. Inflamm Res. 2004. PMID: 15550996 - Nitric Oxide Synthesis Inhibition and Role of P-selectin in Leukocyte Adhesion to Vascular Tissues.
Yang BC, Mehta P, Mehta JL. Yang BC, et al. J Cardiovasc Pharmacol Ther. 1997 Apr;2(2):107-114. doi: 10.1177/107424849700200204. J Cardiovasc Pharmacol Ther. 1997. PMID: 10684448 - Leukocyte-endothelial cell interactions are enhanced in dermal postcapillary venules of MRL/fas(lpr) (lupus-prone) mice: roles of P- and E-selectin.
Hickey MJ, Bullard DC, Issekutz A, James WG. Hickey MJ, et al. J Immunol. 2002 May 1;168(9):4728-36. doi: 10.4049/jimmunol.168.9.4728. J Immunol. 2002. PMID: 11971023 - Critical role of P-selectin-dependent rolling in tumor necrosis factor-alpha-induced leukocyte adhesion and extravascular recruitment in vivo.
Månsson P, Zhang XW, Jeppsson B, Johnell O, Thorlacius H. Månsson P, et al. Naunyn Schmiedebergs Arch Pharmacol. 2000 Aug;362(2):190-6. doi: 10.1007/s002100000268. Naunyn Schmiedebergs Arch Pharmacol. 2000. PMID: 10961383
Cited by
- Salmonella Extracellular Polymeric Substances Modulate Innate Phagocyte Activity and Enhance Tolerance of Biofilm-Associated Bacteria to Oxidative Stress.
Hahn MM, Gunn JS. Hahn MM, et al. Microorganisms. 2020 Feb 13;8(2):253. doi: 10.3390/microorganisms8020253. Microorganisms. 2020. PMID: 32070067 Free PMC article. - cGMP modulation therapeutics for sickle cell disease.
Conran N, Torres L. Conran N, et al. Exp Biol Med (Maywood). 2019 Feb;244(2):132-146. doi: 10.1177/1535370219827276. Epub 2019 Jan 28. Exp Biol Med (Maywood). 2019. PMID: 30691292 Free PMC article. Review. - Compartmentalization Is Key in Limiting Nitric Oxide Scavenging by Cell-Free Hemoglobin.
Kim-Shapiro DB, Patel RP. Kim-Shapiro DB, et al. Am J Respir Crit Care Med. 2016 May 15;193(10):1072-4. doi: 10.1164/rccm.201512-2481ED. Am J Respir Crit Care Med. 2016. PMID: 27174473 Free PMC article. No abstract available. - The Role of Neutrophils in Hypertension.
Araos P, Figueroa S, Amador CA. Araos P, et al. Int J Mol Sci. 2020 Nov 12;21(22):8536. doi: 10.3390/ijms21228536. Int J Mol Sci. 2020. PMID: 33198361 Free PMC article. Review. - Diversity in mechanisms of endothelium-dependent vasodilation in health and disease.
Durand MJ, Gutterman DD. Durand MJ, et al. Microcirculation. 2013 Apr;20(3):239-47. doi: 10.1111/micc.12040. Microcirculation. 2013. PMID: 23311975 Free PMC article. Review.
References
- Petri B, Phillipson M, Kubes P. The physiology of leukocyte recruitment: an in vivo perspective. J Immunol. 2008;180:6439–6446. - PubMed
- Jadert C, Petersson J, Massena S, Ahl D, Grapensparr L, Holm L, Decreased leukocyte recruitment by inorganic nitrate and nitrite in microvascular inflammation and NSAID-induced intestinal injury. Free Radic Biol Med, ; 2011. - PubMed
LinkOut - more resources
Full Text Sources