Emerging roles of non-coding RNAs in brain evolution, development, plasticity and disease - PubMed (original) (raw)
Review
Emerging roles of non-coding RNAs in brain evolution, development, plasticity and disease
Irfan A Qureshi et al. Nat Rev Neurosci. 2012.
Abstract
Novel classes of small and long non-coding RNAs (ncRNAs) are being characterized at a rapid pace, driven by recent paradigm shifts in our understanding of genomic architecture, regulation and transcriptional output, as well as by innovations in sequencing technologies and computational and systems biology. These ncRNAs can interact with DNA, RNA and protein molecules; engage in diverse structural, functional and regulatory activities; and have roles in nuclear organization and transcriptional, post-transcriptional and epigenetic processes. This expanding inventory of ncRNAs is implicated in mediating a broad spectrum of processes including brain evolution, development, synaptic plasticity and disease pathogenesis.
Figures
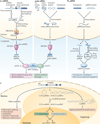
Figure 1. Emerging classes of non-coding RNAs
a | Long non-coding RNAs (lncRNAs; shown in blue) mediate a broad array of genomic and cellular functions and are independently transcribed from: intergenic regions; in antisense, overlapping, intronic and bidirectional orientations to protein-coding genes (black); from gene-regulatory regions, including gene promoters, enhancers and untranslated regions (UTRs); and from specific chromosomal regions, including telomeres (arrows indicate direction of transcription). b | The mitochondrial genome contains small ncRNAs (such as ribosomal RNA (rRNA), tRNA and others) and lncRNAs transcribed from both heavy (outer) and light (inner) strands. Cytochrome b (Cyt b) and NADH dehydrogenase 5 (ND5) and ND6 are mitochondrial protein-coding genes that are associated with mitochondrial lncRNAs transcribed from the complementary mitochondrial DNA (mtDNA) strand. c | Small ncRNAs mediate RNAi and act as guides for RNA modifications (see FIG. 2), and additional small ncRNAs are derived from protein-coding gene (black) regulatory regions and gene boundaries, including 5′ regulatory regions (red), gene termini (orange), intron–exon junctions (yellow) and introns (green). They are also derived from structural components of chromosomes, including centromeres (purple) and telomeres (blue), and can be the cleavage products of other ncRNAs or originate from other sources (white). KERV, kangaroo endogenous retrovirus; NF90, nuclear factor 90; snoRNA, small nucleolar RNA; sRNAs, small RNAs.
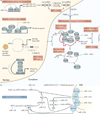
Figure 2. Non-coding RNAs mediating RNAi and RNA modifications
Biogenesis and functions of non-coding RNAs mediating RNAi are shown. a | Left panel: microRNAs (miRNAs) are transcribed as primary miRNA (pri-miRNA; canonical pathway) or from introns (mirtrons; non-canonical pathway). After pri-miRNA and mirtron processing by the DROSHA–DiGeorge syndrome critical region 8 (DGCR8) complex or lariat debranching or folding enzymes, respectively, and nuclear export, precursor miRNAs (pre-miRNAs) are processed by DICER1 and undergo ribonucleoprotein (RNP) assembly with Argonaute proteins 1–4 (AGO1–4). Right panel: endogenous small interfering RNAs (endo-siRNAs) are generated from _cis_- or _trans_-acting sense–antisense pairs, inverted repeats and transposons. endo-siRNAs undergo nuclear processing and export, cytoplasmic processing by DICER2, and RNP assembly with AGO2. miRNAs promote deadenylation and translational repression and endo-siRNAs promote endonucleotytic cleavage, respectively. b | PIWI-interacting RNAs (piRNAs) are generated from transposons and piRNA clusters. piRNA precursors undergo nuclear processing and export, primary or cyclic secondary processing (by the PIWI proteins MILI and MIWI2) and piRNP complex assembly. piRNAs mediate transposon modulation and post-transcription regulation. c | Roles of small nucleolar RNAs (snoRNAs) are shown. snoRNAs are transcribed from introns, processed into C/D and H/ACA snoRNAs, undergo small nucleolar ribonucleoprotein (snoRNP) assembly in Cajal bodies and promote methylation and pseudouridylation of pre-ribosomal RNA (pre-rRNA) in the nucleolus. Small Cajal body-specific RNAs (scaRNAs) promote similar splicesosomal RNA modifications and other snoRNAs display additional functions.
Figure 3. Non-coding RNA dynamics mediate diverse nervous system processes and neurological disease states
Non-coding RNAs (ncRNAs) engage in a complex range of molecular and cellular functions (shown in the pink shaded box). They are also highly integrated, at multiple levels, into the mechanisms and circuitry that underlie neurobiological processes in health and disease. The expression of microRNAs, long ncRNAs (lncRNAs) and possibly other classes of ncRNAs, is similar to the expression of protein-coding genes: it is subject to developmental, constitutive and activity-dependent regulation (yellow lightning bolt) by key modulators of neural gene transcription (including repressor element 1 (RE1)-silencing transcription factor (REST), REST co-repressor 1 (CoREST)) and cAMP response element-binding (CREB)). In turn, ncRNAs influence the expression of these and other seminal neural factors via gene silencing, forming bidirectional regulatory relationships (curved lines, figure right), and by affecting their post-transcriptional RNA processing (alternative splicing (REST4)). ncRNAs can also influence the activity and deployment of neural factors. For example, the small modulatory ncRNA double-stranded neuron-restrictive silencer element (dsNRSE) (red waves) associates with and regulates the REST complex and its modular cofactors (grey ovals), and lncRNAs (red strings) promote the genome-wide deployment of chromatin-modifying complexes (including REST, PRC2 (Polycomb repressor complex 2) and CoREST–REST) (grey arrows). CRE, cAMP response element; NSC, neural stem cell.
Figure 4. Role of non-coding RNAs in synaptic plasticity
a | Diverse non-coding RNA (ncRNA) mechanisms are involved in activity-dependent processes underlying synaptic plasticity (shown in pink shaded boxes). MicroRNAs (miRNAs) contribute to synaptic plasticity through transcription of primary miRNA (pri-miRNA) clusters; processing of pri-miRNAs (by DROSHA and DiGeorge syndrome critical region 8 (DGCR8)) and pre-miRNAs (by DICER1); RNA editing; ribonucleoprotein (RNP) assembly, transport and remodelling; translational repression and activation; and associated mRNA storage, cleavage and degradation in P-bodies. miRNAs also modulate transcription of pri-miRNA clusters and protein-coding plasticity genes through regulation of activity-dependent transcription factors (including cAMP response element-binding (CREB) and myocyte enhancer factor 2 (MEF2)) and methyl-CpG binding proteins (including methyl-CpG-binding protein 2 (MECP2)). The long ncRNAs, brain cytoplasmic RNA 1 (Bc1) and its primate analogue BC200 (red), promote translational repression by binding to RNP components (including eukaryotic translation initiation factor 4A/B (elF4A/B), fragile X mental retardation 1 protein (FMRP) and polyadenylate binding protein (PABP)). Nuclear-enriched abundant transcript 2 (NEAT2) forms nuclear speckles, which are nuclear domains that regulate transcription of plasticity genes (such as, neuroligin 1 (NLGN1) and synaptic cell adhesion molecule 1 (SynCAM1)) and alternative splicing of plasticity genes (such as calcium/calmodulin-dependent protein kinase type II-β (CAMK2B)). Activity-dependent enhancer RNAs (eRNAs) promote transcription of plasticity genes through enhancer–promoter interactions. b | Within the spine head and shaft, modulation of local protein synthesis of neurotransmitter receptors, scaffolding proteins, calcium-dependent signalling molecules and actin cytoskeletal remodelling factors by miRNAs, endogenous small interfering RNAs (endo-siRNAs) and PIWI-interacting RNAs (piRNAs) contributes to synaptic plasticity by regulating spine morphology and synaptic transmission. ADF, actin-depolymerizing factor (also known as gelosin); AGO2, Argonaute 2; APT1, acyl-protein thioesterase 1; CBP, CREB-binding protein; CoREST, REST co-repressor 1; CPEB, cytoplasmic polyadenylation element-binding protein; EPHA4, Eph receptor A4; GluA2, AMPA-type glutamate receptor subunit 2 (also known as GluR2); Gα13, guanine nucleotide-binding protein-α 13; LIMK1, LIM domain kinase 1; MOV10, Moloney leukaemia virus 10 (also known as putative helicase MOV-10); mSin3, paired amphipathic helix family proteins; NR2, glutamate receptor, ionotropic, _N_-methyl d-aspartate 2; PAK, p21 protein (CDC42/RAC)-activated kinase; PSD, postsynaptic density; RAC1, Ras-related C3 botulinum toxin substrate 1; REST, repressor element 1-silencing transcription factor; RHOA, RAS homologue family member A; RISC, RNA-induced silencing complex; SYNGAP1, synaptic RAS GTPase-activating protein 1.
Similar articles
- Noncoding RNAs in the brain.
Satterlee JS, Barbee S, Jin P, Krichevsky A, Salama S, Schratt G, Wu DY. Satterlee JS, et al. J Neurosci. 2007 Oct 31;27(44):11856-9. doi: 10.1523/JNEUROSCI.3624-07.2007. J Neurosci. 2007. PMID: 17978024 Free PMC article. Review. - Non-coding RNAs with essential roles in neurodegenerative disorders.
Salta E, De Strooper B. Salta E, et al. Lancet Neurol. 2012 Feb;11(2):189-200. doi: 10.1016/S1474-4422(11)70286-1. Lancet Neurol. 2012. PMID: 22265214 Review. - Diverse functions of nuclear non-coding RNAs in eukaryotic gene expression.
Chinen M, Tani T. Chinen M, et al. Front Biosci (Landmark Ed). 2012 Jan 1;17(4):1402-17. doi: 10.2741/3994. Front Biosci (Landmark Ed). 2012. PMID: 22201811 - Mechanisms of Long Non-coding RNAs in Mammalian Nervous System Development, Plasticity, Disease, and Evolution.
Briggs JA, Wolvetang EJ, Mattick JS, Rinn JL, Barry G. Briggs JA, et al. Neuron. 2015 Dec 2;88(5):861-877. doi: 10.1016/j.neuron.2015.09.045. Neuron. 2015. PMID: 26637795 Review. - The RNA-centred view of the synapse: non-coding RNAs and synaptic plasticity.
Smalheiser NR. Smalheiser NR. Philos Trans R Soc Lond B Biol Sci. 2014 Sep 26;369(1652):20130504. doi: 10.1098/rstb.2013.0504. Philos Trans R Soc Lond B Biol Sci. 2014. PMID: 25135965 Free PMC article. Review.
Cited by
- Intracerebral hemorrhage alters circular RNA expression profiles in the rat brain.
Zhong Y, Li X, Li C, Li Y, He Y, Li F, Ling L. Zhong Y, et al. Am J Transl Res. 2020 Aug 15;12(8):4160-4174. eCollection 2020. Am J Transl Res. 2020. PMID: 32913495 Free PMC article. - Epigenetics and therapeutic targets mediating neuroprotection.
Qureshi IA, Mehler MF. Qureshi IA, et al. Brain Res. 2015 Dec 2;1628(Pt B):265-272. doi: 10.1016/j.brainres.2015.07.034. Epub 2015 Jul 30. Brain Res. 2015. PMID: 26236020 Free PMC article. Review. - Long noncoding RNA CCDC144NL-AS1 knockdown induces naïve-like state conversion of human pluripotent stem cells.
Wang Y, Guo B, Xiao Z, Lin H, Zhang X, Song Y, Li Y, Gao X, Yu J, Shao Z, Li X, Luo Y, Li S. Wang Y, et al. Stem Cell Res Ther. 2019 Jul 29;10(1):220. doi: 10.1186/s13287-019-1323-9. Stem Cell Res Ther. 2019. PMID: 31358062 Free PMC article. - Long noncoding RNA H19 in the injured dorsal root ganglion contributes to peripheral nerve injury-induced pain hypersensitivity.
Wen J, Yang Y, Wu S, Wei G, Jia S, Hannaford S, Tao YX. Wen J, et al. Transl Perioper Pain Med. 2020;7(2):176-184. Epub 2020 Jan 18. Transl Perioper Pain Med. 2020. PMID: 32099850 Free PMC article. - Epigenetic mechanisms underlying nervous system diseases.
Qureshi IA, Mehler MF. Qureshi IA, et al. Handb Clin Neurol. 2018;147:43-58. doi: 10.1016/B978-0-444-63233-3.00005-1. Handb Clin Neurol. 2018. PMID: 29325627 Free PMC article. Review.
References
- Birney E, et al. Identification and analysis of functional elements in 1% of the human genome by the ENCODE pilot project. Nature. 2007;447:799–816. This paper reported the results of the pilot phase of the ENCODE project, which aims to define the regulatory and functional landscape of the human genome, and provided evidence supporting the conclusion that the human genome is pervasively transcribed.
- Carninci P, et al. The transcriptional landscape of the mammalian genome. Science. 2005;309:1559–1563. - PubMed
- Katayama S, et al. Antisense transcription in the mammalian transcriptome. Science. 2005;309:1564–1566. References and reported data from the FANTOM3 project, which focused on characterizing the mammalian transcriptome and the organization of transcriptional and regulatory units in the genome, and identified the diversity of protein-coding and ncRNA transcripts that arise from overlapping transcription on both strands.
- White RJ. Transcription by RNA polymerase III: more complex than we thought. Nature Rev. Genet. 2011;12:459–463. - PubMed
- Candeias MM. The can and can’t dos of p53. RNA. Biochimie. 2011;93:1962–1965. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- NS071571/NS/NINDS NIH HHS/United States
- R01 MH066290/MH/NIMH NIH HHS/United States
- MH66290/MH/NIMH NIH HHS/United States
- P30 HD001799/HD/NICHD NIH HHS/United States
- R01 NS071571/NS/NINDS NIH HHS/United States
- HD071593/HD/NICHD NIH HHS/United States
- P30 HD071593/HD/NICHD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical