BRAG2/GEP100/IQSec1 interacts with clathrin and regulates α5β1 integrin endocytosis through activation of ADP ribosylation factor 5 (Arf5) - PubMed (original) (raw)
BRAG2/GEP100/IQSec1 interacts with clathrin and regulates α5β1 integrin endocytosis through activation of ADP ribosylation factor 5 (Arf5)
Radim Moravec et al. J Biol Chem. 2012.
Abstract
ADP ribosylation factors (Arfs) are small GTP-binding proteins known for their role in vesicular transport, where they nucleate the assembly of coat protein complexes at sites of carrier vesicle formation. Similar to other GTPases, Arfs require guanine nucleotide exchange factors to catalyze GTP loading and activation. One subfamily of ArfGEFs, the BRAGs, has been shown to activate Arf6, which acts in the endocytic pathway to control the trafficking of a subset of cargo proteins including integrins. We have previously shown that BRAG2 modulates cell adhesion by regulating integrin surface expression. Here, we show that, in addition to Arf6, endogenous BRAG2 also activates the class II Arfs, Arf4 and Arf5, and that surprisingly, it is Arf5 that mediates integrin internalization. We observed that cell spreading on fibronectin is enhanced upon inhibition of BRAG2 or Arf5 but not Arf6. Similarly, spreading in BRAG2-depleted cells is reverted by expression of a rapid cycling Arf5 mutant (T161A) but not by a corresponding Arf6 construct (T157A). We also show that BRAG2 binds clathrin and the AP-2 adaptor complex and that both BRAG2 and Arf5 localize to clathrin-coated pits at the plasma membrane. Consistent with these observations, depletion of Arf5, but not Arf6 or Arf4, slows internalization of β1 integrins without affecting transferrin receptor uptake. Together, these findings indicate that BRAG2 acts at clathrin-coated pits to promote integrin internalization by activating Arf5 and suggest a previously unrecognized role for Arf5 in clathrin-mediated endocytosis of specific cargoes.
Figures
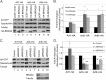
FIGURE 1.
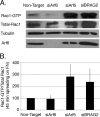
BRAG2 activates Arf1, Arf5, and Arf6. A, HeLa cells were transfected overnight with plasmids encoding C-terminally tagged Arf1-HA, Arf5-HA, or Arf6-HA in the presence of either empty vector (pCB7-vector), HA-BRAG2a, or HA-BRAG2b to stimulate Arf activation. Cell lysates were incubated with GST-GGA3 to precipitate active GTP-bound Arf. The combined results of seven independent experiments are quantified in B. For each Arf, basal activity (in the absence of BRAG2) was normalized to a value of 1, and data are expressed as fold activation (±S.E.) relative to control. C, HeLa cells treated with non-target RNAi (control) or BRAG2 oligonucleotides (siBRAG2–1 and siBRAG2–2) were transfected as described above with either Arf1-HA, Arf5-HA, or Arf6-HA plasmids to determine changes in Arf activity after depletion of endogenous BRAG2. Results are quantified in D (n = 3). Using a t test, p < 0.05 is denoted by a single asterisk, and p < 0.005 is denoted by a double asterisk.
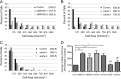
FIGURE 2.
BRAG2 and Arf5 regulate cell spreading. Cells were depleted of endogenous BRAG2 (A), Arf5 (B), or Arf6 (C) using two individual siRNA oligonucleotides for each target. Cells were then suspended and replated on FN-coated coverslips (5 μg/ml) for 1.5 h to allow spreading. Cells were then fixed and stained with rhodamine phalloidin to visualize actin. Random fields were imaged, and the surface area of each cell was measured for 100 cells per condition. Because surface area varied considerably among cells in each population, measurements were binned to more accurately indicate variance. Mean surface area for each population is indicated in parentheses for each condition (A–C). Mean cell areas from three replicates (n = 3) of each experiment were averaged to generate D, and a one-way parametric analysis of variance (with Bonferroni's multiple comparison test) was performed to determine statistical significance between the indicated populations (*, p < 0.05; **, p < 0.005). Cont, control; NS, not significant.
FIGURE 3.
Arf5 but not Arf6 restores normal spreading in BRAG2-depleted cells. A, surface area of spread cells was measured for control (mock-depleted) or BRAG2-depleted cells as in Fig. 2. B, BRAG2-depleted cells were transfected with plasmids encoding rapid-cycling mutants of either Arf5-HA (Arf5-RC) or Arf6-HA (Arf6-RC), and spreading was measured as described in A. C, neither rapid cycling Arf5 nor Arf6 affects spreading in control, non-BRAG2-depleted cells. Surface areas of spread cells were measured as indicated in the legend to Fig. 2. D, mean surface areas ± S.E. were calculated for each condition from three replicate (n = 3) experiments. A one-way parametric analysis of variance (with Bonferroni's multiple comparison test) was performed to demonstrate statistical significance, where p < 0.0005 are denoted by ***; and p < 0.00001 are denoted by ****. E, relative binding of wild-type Arf5-HA or Arf5-T161A-HA rapid cycling mutant to GST-GGA3 was determined in a pulldown assay. F, a representative immunoblot showing the extent of BRAG2 depletion and relative expression of rapid-cycling Arf5 and Arf6. NS, not significant.
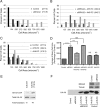
FIGURE 4.
BRAG2 and Arf5 regulate β1 integrin endocytosis. Cells depleted of BRAG2, Arf5, Arf6, or clathrin were stained with antibody specific for β1 integrin (A), α5 integrin (B), or transferrin receptor (Trf-R; F), and the amount of surface-bound antibody in each sample was detected by flow cytometry. Values are normalized to control (mock-depleted) cells. C and D, internalization of β1 integrin (C) or transferrin receptor (D) antibody/receptor complexes was measured by flow cytometry (n = 3) as described under “Experimental Procedures.” E, total β1 integrin and transferrin receptor expression in clathrin-, Arf-, or BRAG2-depleted cells was measured by immunoblot. F, cell surface transferrin receptor was measured by flow cytometry (n = 3 for each condition). Using a t test, p < 0.05 is denoted by *; and p < 0.005 is denoted by **.
FIGURE 5.
Arf6 but not Arf5 regulates Rac1 activation during cell spreading. A, cells depleted of Arf6, Arf5, or BRAG2 were plated on FN-coated coverslips for 45 min in serum-free DMEM to allow spreading, and Rac1 activation was measured using a standard PBD pulldown assay as described under “Experimental Procedures.” B, quantification of the ratio of Rac1-GTP to total Rac1 (n = 3). Values are normalized to control cells treated with non-targeting siRNA.
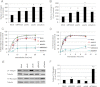
FIGURE 6.
BRAG2 interacts with clathrin in vitro. A, domain organization of BRAG2a and BRAG2b (GenBankTM accession numbers JF432314 and NM_014869.5). Positions of clathrin boxes are indicated. B, alignment of clathrin boxes from BRAG2 with related sequences from other clathrin-binding proteins. C, HeLa cell lysates were incubated with either GST alone (GST), GST-fusion proteins containing BRAG2 clathrin box 1 (CB1), or clathrin box 2 (CB2). The resulting precipitates were immunoblotted for clathrin heavy chain and β-adaptin. In lanes 4–6, cells were depleted of clathrin by siRNA to demonstrate clathrin-independent binding of β-adaptin to BRAG2. Ponceau staining was used to demonstrate equal levels of GST fusion proteins in each sample. D, quantitation of the results shown in C (n = 6). E, HeLa cell lysates or lysis buffer alone were incubated with GST or GST-CB2, and precipitates were immunoblotted for clathrin heavy chain, γ-adaptin (AP-1), or β-adaptin (AP-2). Note that only β-adaptin co-precipitates with GST-CB2.
FIGURE 7.
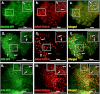
BRAG2, Arf5, and Arf6 localize to clathrin-coated pits at the plasma membrane. HeLa cells plated on FN (5 μg/ml) were transfected with plasmids encoding dsRed-clathrin and either BRAG2b-GFP (A–C), Arf5-GFP (D–F), or Arf6-GFP (G–I). Live cells were imaged using TIR-FM. Areas indicated with dashed boxes are enlarged and shown as insets. Arrows indicate colocalization with clathrin.
Similar articles
- The Arf6 GEF GEP100/BRAG2 regulates cell adhesion by controlling endocytosis of beta1 integrins.
Dunphy JL, Moravec R, Ly K, Lasell TK, Melancon P, Casanova JE. Dunphy JL, et al. Curr Biol. 2006 Feb 7;16(3):315-20. doi: 10.1016/j.cub.2005.12.032. Curr Biol. 2006. PMID: 16461286 Free PMC article. - BRAG2a Mediates mGluR-Dependent AMPA Receptor Internalization at Excitatory Postsynapses through the Interaction with PSD-95 and Endophilin 3.
Fukaya M, Sugawara T, Hara Y, Itakura M, Watanabe M, Sakagami H. Fukaya M, et al. J Neurosci. 2020 May 27;40(22):4277-4296. doi: 10.1523/JNEUROSCI.1645-19.2020. Epub 2020 Apr 27. J Neurosci. 2020. PMID: 32341099 Free PMC article. - Brag2 differentially regulates β1- and β3-integrin-dependent adhesion in endothelial cells and is involved in developmental and pathological angiogenesis.
Manavski Y, Carmona G, Bennewitz K, Tang Z, Zhang F, Sakurai A, Zeiher AM, Gutkind JS, Li X, Kroll J, Dimmeler S, Chavakis E. Manavski Y, et al. Basic Res Cardiol. 2014 Mar;109(2):404. doi: 10.1007/s00395-014-0404-2. Epub 2014 Feb 13. Basic Res Cardiol. 2014. PMID: 24522833 - Phosphoinositide regulation of clathrin-mediated endocytosis.
Haucke V. Haucke V. Biochem Soc Trans. 2005 Dec;33(Pt 6):1285-9. doi: 10.1042/BST0331285. Biochem Soc Trans. 2005. PMID: 16246100 Review. - The EGFR-GEP100-Arf6-AMAP1 signaling pathway specific to breast cancer invasion and metastasis.
Sabe H, Hashimoto S, Morishige M, Ogawa E, Hashimoto A, Nam JM, Miura K, Yano H, Onodera Y. Sabe H, et al. Traffic. 2009 Aug;10(8):982-93. doi: 10.1111/j.1600-0854.2009.00917.x. Epub 2009 Apr 21. Traffic. 2009. PMID: 19416474 Free PMC article. Review.
Cited by
- The Arf6 GTPase-activating proteins ARAP2 and ACAP1 define distinct endosomal compartments that regulate integrin α5β1 traffic.
Chen PW, Luo R, Jian X, Randazzo PA. Chen PW, et al. J Biol Chem. 2014 Oct 31;289(44):30237-30248. doi: 10.1074/jbc.M114.596155. Epub 2014 Sep 15. J Biol Chem. 2014. PMID: 25225293 Free PMC article. - Integrin traffic - the update.
De Franceschi N, Hamidi H, Alanko J, Sahgal P, Ivaska J. De Franceschi N, et al. J Cell Sci. 2015 Mar 1;128(5):839-52. doi: 10.1242/jcs.161653. Epub 2015 Feb 6. J Cell Sci. 2015. PMID: 25663697 Free PMC article. Review. - Allosteric properties of PH domains in Arf regulatory proteins.
Roy NS, Yohe ME, Randazzo PA, Gruschus JM. Roy NS, et al. Cell Logist. 2016 Apr 26;6(2):e1181700. doi: 10.1080/21592799.2016.1181700. eCollection 2016 Apr-Jun. Cell Logist. 2016. PMID: 27294009 Free PMC article. Review. - Artificial-Intelligence-Assisted Discovery of Genetic Factors for Precision Medicine of Antiplatelet Therapy in Diabetic Peripheral Artery Disease.
Yeh CH, Chou YJ, Tsai TH, Hsu PW, Li CH, Chan YH, Tsai SF, Ng SC, Chou KM, Lin YC, Juan YH, Fu TC, Lai CC, Sytwu HK, Tsai TF. Yeh CH, et al. Biomedicines. 2022 Jan 6;10(1):116. doi: 10.3390/biomedicines10010116. Biomedicines. 2022. PMID: 35052795 Free PMC article. - Calcium-stimulated disassembly of focal adhesions mediated by an ORP3/IQSec1 complex.
D'Souza RS, Lim JY, Turgut A, Servage K, Zhang J, Orth K, Sosale NG, Lazzara MJ, Allegood J, Casanova JE. D'Souza RS, et al. Elife. 2020 Apr 1;9:e54113. doi: 10.7554/eLife.54113. Elife. 2020. PMID: 32234213 Free PMC article.
References
- Robinson M. S. (2004) Adaptable adaptors for coated vesicles. Trends Cell Biol. 14, 167–174 - PubMed
- Chun J., Shapovalova Z., Dejgaard S. Y., Presley J. F., Melançon P. (2008) Characterization of class I and II ADP-ribosylation factors (Arfs) in live cells: GDP-bound class II Arfs associate with the ER-Golgi intermediate compartment independently of GBF1. Mol. Biol. Cell 19, 3488–3500 - PMC - PubMed
- D'Souza-Schorey C., Chavrier P. (2006) ARF proteins: Roles in membrane traffic and beyond. Nat. Rev. Mol. Cell Biol. 7, 347–358 - PubMed
- Casanova J. E. (2007) Regulation of Arf activation: The Sec7 family of guanine nucleotide exchange factors. Traffic 8, 1476–1485 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous