Inactivation of basolateral amygdala specifically eliminates palatability-related information in cortical sensory responses - PubMed (original) (raw)
Inactivation of basolateral amygdala specifically eliminates palatability-related information in cortical sensory responses
Caitlin E Piette et al. J Neurosci. 2012.
Abstract
Evidence indirectly implicates the amygdala as the primary processor of emotional information used by cortex to drive appropriate behavioral responses to stimuli. Taste provides an ideal system with which to test this hypothesis directly, as neurons in both basolateral amygdala (BLA) and gustatory cortex (GC)-anatomically interconnected nodes of the gustatory system-code the emotional valence of taste stimuli (i.e., palatability), in firing rate responses that progress similarly through "epochs." The fact that palatability-related firing appears one epoch earlier in BLA than GC is broadly consistent with the hypothesis that such information may propagate from the former to the latter. Here, we provide evidence supporting this hypothesis, assaying taste responses in small GC single-neuron ensembles before, during, and after temporarily inactivating BLA in awake rats. BLA inactivation (BLAx) changed responses in 98% of taste-responsive GC neurons, altering the entirety of every taste response in many neurons. Most changes involved reductions in firing rate, but regardless of the direction of change, the effect of BLAx was epoch-specific: while firing rates were changed, the taste specificity of responses remained stable; information about taste palatability, however, which normally resides in the "Late" epoch, was reduced in magnitude across the entire GC sample and outright eliminated in most neurons. Only in the specific minority of neurons for which BLAx enhanced responses did palatability specificity survive undiminished. Our data therefore provide direct evidence that BLA is a necessary component of GC gustatory processing, and that cortical palatability processing in particular is, in part, a function of BLA activity.
Figures
Figure 1.
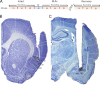
Experimental timeline and electrode/cannula placement. A, Timeline of the experimental protocol showing the three recording sessions (red) and time of interpolated infusion (Inf., blue). B, Sample histology showing placement of electrode tips. X's and O's denote the locations of the electrode tips for muscimol-treated and control animals, respectively. C, Sample histology showing placement of cannula tips centered in BLA. AID, Agranular insular cortex (dorsal); AIV, agranular insular cortex (ventral); BLP, basolateral amygdala (posterior); BLV, basolateral amygdala (ventral); BMP, basomedial amygdala (posterior); DI, dysgranular insular cortex; GI, granular insular cortex; LaVM, lateral amygdaloid nucleus (ventromedial); Pir, piriform cortex; S1, primary somatosensory cortex; STIA, strial terminalis. Schematic outlines adapted from Paxinos and Watson, 2007; used with permission.
Figure 2.
Taste responses can be observed in GC. A, The top half shows raster plots of spiking activity of a single representative GC neuron in response to presentation of the four basic tastes (sucrose, green; quinine, black; citric acid, red; NaCl, pink). Each row is a single trial, and each dot is an action potential. Below are the corresponding PSTHs, which show the firing rate in spikes/second (_y_-axis) of the neuron's response to taste; _x_-axis, poststimulus time in milliseconds. Zero is the moment of taste delivery, marked with a vertical dashed line. B, A second representative neuron's response to the same tastes. C, The percentage of GC neurons (_y_-axis) that are taste-responsive (gray), taste-selective (black), and palatability-related (dashed) when BLA is intact (_x_-axis, poststimulus time in ms). Because each trace reflects an analysis involving a sliding window, some smoothing has necessarily occurred. D, The number of neurons (_y_-axis) responding to each of the four tastes (_x_-axis) during the Intact Middle and Late epochs; somewhat fewer neurons respond to each taste in the Late epoch. E, The number of neurons (_y_-axis) that responded to a particular number of taste stimuli (_x_-axis) during the Intact Middle and Late epochs.
Figure 3.
BLAx changes firing rate of responses. A, Representative example of how a GC neuron's responses to tastes in the Intact session (left PSTHs) could be altered following muscimol infusion into BLA—in this case, BLAx ubiquitously decreased responses (right PSTHs). Same conventions as Figure 2. B, A second example GC neuron's responses, showing (less common) firing-rate increases wrought by BLAx.
Figure 4.
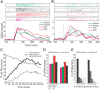
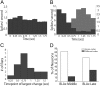
The overall impact of BLAx on the entire GC population. A, Pie chart showing how different GC neurons were affected by BLAx. For 65% of the neurons, responses to all 4 tastes changed (blue); for 55.9% of these, responses were either all decreased (38.2%, dark blue) or all increased (17.7%, light blue; hatching shows the few single neurons for which some changes were reductions and others enhancements). BLAx changed responses to 3 of 4 tastes for 12% of the remaining GC neurons (purple), changed responses to 2 tastes in 16% (green), etc. B, Graph showing the firing rate in spikes/second (_y_-axis) for all tastes in the Middle and Late epochs (_x_-axis) in both the BLA Intact (left) and BLAx (right) sessions. Within a single taste, the difference in firing rates between the Middle and Late epochs within a session was significant (p < 0.05 by t test). The difference in firing rate within a single taste was likewise significant (p < 0.05 by t test) within an epoch across sessions. C, The result of a classification analysis, showing that taste identity discrimination performance—the ability to correctly identify (percentage correct, _y_-axis) the administered taste (top)—was similarly high in Intact (left) and BLAx (right) sessions, across the overall GC population. Along the _x_-axis for each set of bars is the algorithm's identification choice; chance performance (25%) is shown with the horizontal dashed line.
Figure 5.
Taste responses recover within 8 h after muscimol infusion. A, PSTHs (same conventions) for a representative GC neuron, showing responses increasing following BLAx and recovering within 8 h; the insets show corresponding waveforms (black) with average noise trace (red). B, PSTH of second representative neuron for which responses decreased following BLAx and recovered within 8 h.
Figure 6.
Epoch dependency of the effects of BLAx. A, Absolute differences in firing rates (spikes/s, _y_-axis) between Intact and BLAx sessions for the entire sample (n = 145) of GC neurons across time in seconds (_x_-axis), divided into 250 ms bins. B, Similar histograms for representative ensembles from two animals demonstrating time-structure that is lost in the overall average; for one ensemble (dark gray), BLAx-induced changes measured in terms of absolute differences in firing rates were high early and lower late; for the other example (light gray), the pattern was reversed. The _x_- and _y_-axes are the same as for A. C, The results of change point analysis brought to bear on the data for each ensemble, showing that the impact of BLAx reliably changed between 0.75 and 1 s (bins 3 and 4). _y_-axis, Number of ensembles for which the greatest change in impact of BLAx occurred between particular pairs of bins; _x_-axis, time point of the largest change in seconds. D, The percentage of neurons (_y_-axis) where the order of responsiveness (e.g., which tastes caused the largest, second largest, third largest, and smallest responses) was the same (open) during BLAx as BLA Intact or different (gray) in both the Middle and Late epochs (_x_-axis).
Figure 7.
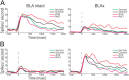
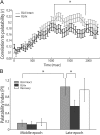
Late-epoch palatability processing is specifically affected by BLAx. A, Moving-window analysis of the correlation between firing rates and taste palatability (_y_-axis) across poststimulus time (_x_-axis), performed separately for Intact (light gray) and BLAx (dark gray) sessions. As expected, the correlations rise to a peak only at the end of the first second (moving-window procedure “smoothes” the data such that the correlation appears to rise earlier than it truly does). Note that the functions for Intact and BLAx diverge in the last half of the first second; the palatability correlation is significantly lower for BLAx starting at 1.1 s. B, Summary of this change in palatability processing, using a PI (_y_-axis) defined as the difference between the mean firing rate responses to tastes with similar and different palatabilities, computed separately for Intact (light gray), BLAx (dark gray), and Recovery (open) sessions. Note that the actual amount of palatability-related activity is close to zero (i.e., no palatability-related response) in the Middle epoch. The expected difference in palatability content between Middle and Late epochs during Intact sessions (light gray) is significant, as is the comparison between the Intact and BLAx Late epoch PI. For both panels, error bars represent SEM and *p < 0.05.
Figure 8.
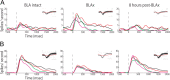
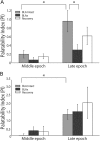
Palatability processing is not affected by neurons with increasing firing rate post-BLAx. A, PI analysis (conventions same as Fig. 7) for the specific subgroup of neurons for which response magnitudes were reduced by BLAx. The growth in palatability content from the Middle to the Late epoch is significant for Intact sessions, but not for BLAx sessions. Within the Late epoch, there is a significant difference between Intact and BLAx. B, When analysis is restricted to the subgroup of neurons for which responses are enhanced BLAx, the significant palatability-related difference between Intact and BLAx sessions in the Late epoch disappears. Note the difference in _y_-axis scaling for the two panels. *p < 0.05.
Similar articles
- Sodium concentration coding gives way to evaluative coding in cortex and amygdala.
Sadacca BF, Rothwax JT, Katz DB. Sadacca BF, et al. J Neurosci. 2012 Jul 18;32(29):9999-10011. doi: 10.1523/JNEUROSCI.6059-11.2012. J Neurosci. 2012. PMID: 22815514 Free PMC article. - Perturbation of amygdala-cortical projections reduces ensemble coherence of palatability coding in gustatory cortex.
Lin JY, Mukherjee N, Bernstein MJ, Katz DB. Lin JY, et al. Elife. 2021 May 21;10:e65766. doi: 10.7554/eLife.65766. Elife. 2021. PMID: 34018924 Free PMC article. - Coupled Dynamics of Stimulus-Evoked Gustatory Cortical and Basolateral Amygdalar Activity.
Mahmood A, Steindler J, Germaine H, Miller P, Katz DB. Mahmood A, et al. J Neurosci. 2023 Jan 18;43(3):386-404. doi: 10.1523/JNEUROSCI.1412-22.2022. Epub 2022 Nov 28. J Neurosci. 2023. PMID: 36443002 Free PMC article. - Coding in the mammalian gustatory system.
Carleton A, Accolla R, Simon SA. Carleton A, et al. Trends Neurosci. 2010 Jul;33(7):326-34. doi: 10.1016/j.tins.2010.04.002. Epub 2010 May 20. Trends Neurosci. 2010. PMID: 20493563 Free PMC article. Review. - A comparative analysis of neural taste processing in animals.
de Brito Sanchez G, Giurfa M. de Brito Sanchez G, et al. Philos Trans R Soc Lond B Biol Sci. 2011 Jul 27;366(1574):2171-80. doi: 10.1098/rstb.2010.0327. Philos Trans R Soc Lond B Biol Sci. 2011. PMID: 21690133 Free PMC article. Review.
Cited by
- Scopolamine infusion in the basolateral amygdala after saccharin intake induces conditioned taste avoidance in rats.
Torres-García VM, Rodríguez-Nava E, Alcántara-Rivas RI, Picazo O, Roldán-Roldán G, Morin JP. Torres-García VM, et al. Psychopharmacology (Berl). 2024 Oct;241(10):2133-2144. doi: 10.1007/s00213-024-06624-7. Epub 2024 Jun 1. Psychopharmacology (Berl). 2024. PMID: 38822849 Free PMC article. - Multisensory Integration Underlies the Distinct Representation of Odor-Taste Mixtures in the Gustatory Cortex of Behaving Rats.
Stocke S, Samuelsen CL. Stocke S, et al. J Neurosci. 2024 May 15;44(20):e0071242024. doi: 10.1523/JNEUROSCI.0071-24.2024. J Neurosci. 2024. PMID: 38548337 Free PMC article. - Oral thermal processing in the gustatory cortex of awake mice.
Bouaichi CG, Odegaard KE, Neese C, Vincis R. Bouaichi CG, et al. Chem Senses. 2023 Jan 1;48:bjad042. doi: 10.1093/chemse/bjad042. Chem Senses. 2023. PMID: 37850853 Free PMC article. - Inhibitory Gating of Thalamocortical Inputs onto Rat Gustatory Insular Cortex.
Haley MS, Fontanini A, Maffei A. Haley MS, et al. J Neurosci. 2023 Nov 1;43(44):7294-7306. doi: 10.1523/JNEUROSCI.2255-22.2023. Epub 2023 Sep 13. J Neurosci. 2023. PMID: 37704374 Free PMC article. - Taste-Odor Association Learning Alters the Dynamics of Intraoral Odor Responses in the Posterior Piriform Cortex of Awake Rats.
Maier JX, Idris A, Christensen BA. Maier JX, et al. eNeuro. 2023 Mar 29;10(3):ENEURO.0010-23.2023. doi: 10.1523/ENEURO.0010-23.2023. Print 2023 Mar. eNeuro. 2023. PMID: 36898831 Free PMC article.
References
- Arikan R, Blake NM, Erinjeri JP, Woolsey TA, Giraud L, Highstein SM. A method to measure the effective spread of focally injected muscimol into the central nervous system with electrophysiology and light microscopy. J Neurosci Methods. 2002;118:51–57. - PubMed
- Bahar A, Dudai Y, Ahissar E. Neural signature of taste familiarity in the gustatory cortex of the freely behaving rat. J Neurophysiol. 2004;92:3298–3308. - PubMed
- Bechara A, Damasio H, Damasio AR. Role of the amygdala in decision-making. Ann N Y Acad Sci. 2003;985:356–369. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- DC-011235/DC/NIDCD NIH HHS/United States
- DC-006666/DC/NIDCD NIH HHS/United States
- R01 DC006666/DC/NIDCD NIH HHS/United States
- R01 DC007703/DC/NIDCD NIH HHS/United States
- F31 DC011235/DC/NIDCD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous