Dietary fibre improves first-phase insulin secretion in overweight individuals - PubMed (original) (raw)
Randomized Controlled Trial
Dietary fibre improves first-phase insulin secretion in overweight individuals
Caroline L Bodinham et al. PLoS One. 2012.
Abstract
Previous work has shown increased insulin sensitivity, increased hepatic insulin clearance and lower postprandial insulin responses following treatment with resistant starch, a type of dietary fibre. The objective of this study was to further explore the effects of resistant starch on insulin secretion. Twelve overweight (BMI 28.2±0.4 kg/m(2)) individuals participated in this randomized, subject-blind crossover study. Participants consumed either 40 g type 2 resistant starch or the energy and carbohydrate-matched placebo daily for four weeks. Assessment of the effect on insulin secretion was made at the end of each intervention using an insulin-modified frequently sampled intravenous glucose tolerance test (FSIVGTT). Insulin and C-peptide concentrations were significantly higher during the FSIVGTT following the resistant starch compared with the placebo. Modelling of the data showed significantly improved first-phase insulin secretion with resistant starch. These effects were observed without any changes to either body weight or habitual food intake. This study showed that just four weeks of resistant starch intake significantly increased the first-phase insulin secretion in individuals at risk of developing type 2 diabetes. Further studies exploring this effect in individuals with type 2 diabetes are required.
Conflict of interest statement
Competing Interests: CLB received an educational fellowship from Premier Foods; this fellowship was independent to the research and was not involved in the study design, collection, analysis and interpretation of data, writing of the paper and/or decisions to submit the publication. This does not alter the authors' adherence to all the PLoS ONE policies on sharing data and materials. The other authors have declared that no competing interests exist.
Figures
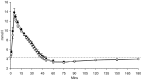
Figure 1. Glucose concentrations from FSIVGTT after 4 weeks daily supplementation with 40 g HAM-RS2 or placebo.
N = 12, mean ± SEM. No significant difference between the HAM-RS2 and placebo. Comparisons made with repeated measures ANOVA. Black circles = HAM-RS2; white circles = placebo; dashed line = baseline glucose concentrations.
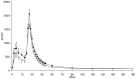
Figure 2. Insulin concentrations from FSIVGTT after 4 weeks daily supplementation with 40 g HAM-RS2 or placebo.
N = 12, mean ± SEM. Significantly higher concentrations following HAM-RS2 compared with placebo (p = 0.009). Comparisons made with repeated measures ANOVA. Black circles = HAM-RS2; white circles = placebo.
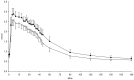
Figure 3. C-peptide concentrations from FSIVGTT after 4 weeks daily supplementation with 40 g HAM-RS2 or placebo.
N = 12, mean ± SEM. Significantly higher concentrations following HAM-RS2 compared with placebo (p = 0.016). Comparisons made with repeated measures ANOVA. Black circles = HAM-RS2; white circles = placebo.
Similar articles
- Acute Effect of Resistant Starch on Food Intake, Appetite and Satiety in Overweight/Obese Males.
Al-Mana NM, Robertson MD. Al-Mana NM, et al. Nutrients. 2018 Dec 15;10(12):1993. doi: 10.3390/nu10121993. Nutrients. 2018. PMID: 30558330 Free PMC article. Clinical Trial. - Resistant starch lowers postprandial glucose and leptin in overweight adults consuming a moderate-to-high-fat diet: a randomized-controlled trial.
Maziarz MP, Preisendanz S, Juma S, Imrhan V, Prasad C, Vijayagopal P. Maziarz MP, et al. Nutr J. 2017 Feb 21;16(1):14. doi: 10.1186/s12937-017-0235-8. Nutr J. 2017. PMID: 28222742 Free PMC article. Clinical Trial. - Acute ingestion of resistant starch reduces food intake in healthy adults.
Bodinham CL, Frost GS, Robertson MD. Bodinham CL, et al. Br J Nutr. 2010 Mar;103(6):917-22. doi: 10.1017/S0007114509992534. Epub 2009 Oct 27. Br J Nutr. 2010. PMID: 19857367 Clinical Trial. - Effects of Higher Dietary Protein and Fiber Intakes at Breakfast on Postprandial Glucose, Insulin, and 24-h Interstitial Glucose in Overweight Adults.
Amankwaah AF, Sayer RD, Wright AJ, Chen N, McCrory MA, Campbell WW. Amankwaah AF, et al. Nutrients. 2017 Apr 2;9(4):352. doi: 10.3390/nu9040352. Nutrients. 2017. PMID: 28368334 Free PMC article. Clinical Trial. - NUTRIOSE dietary fiber supplementation improves insulin resistance and determinants of metabolic syndrome in overweight men: a double-blind, randomized, placebo-controlled study.
Li S, Guerin-Deremaux L, Pochat M, Wils D, Reifer C, Miller LE. Li S, et al. Appl Physiol Nutr Metab. 2010 Dec;35(6):773-82. doi: 10.1139/H10-074. Appl Physiol Nutr Metab. 2010. PMID: 21164548 Clinical Trial.
Cited by
- Metabolic Effects of Resistant Starch Type 2: A Systematic Literature Review and Meta-Analysis of Randomized Controlled Trials.
Snelson M, Jong J, Manolas D, Kok S, Louise A, Stern R, Kellow NJ. Snelson M, et al. Nutrients. 2019 Aug 8;11(8):1833. doi: 10.3390/nu11081833. Nutrients. 2019. PMID: 31398841 Free PMC article. - Could Dietary Modification Independent of Energy Balance Influence the Underlying Pathophysiology of Type 2 Diabetes? Implications for Type 2 Diabetes Remission.
Guess ND. Guess ND. Diabetes Ther. 2022 Apr;13(4):603-617. doi: 10.1007/s13300-022-01220-4. Epub 2022 Mar 10. Diabetes Ther. 2022. PMID: 35266093 Free PMC article. Review. - A Whole-Grain Diet Increases Glucose-Stimulated Insulin Secretion Independent of Gut Hormones in Adults at Risk for Type 2 Diabetes.
Malin SK, Kullman EL, Scelsi AR, Godin JP, Ross AB, Kirwan JP. Malin SK, et al. Mol Nutr Food Res. 2019 Apr;63(7):e1800967. doi: 10.1002/mnfr.201800967. Epub 2019 Mar 20. Mol Nutr Food Res. 2019. PMID: 30763457 Free PMC article. Clinical Trial. - Kappaphycus alvarezii as a Food Supplement Prevents Diet-Induced Metabolic Syndrome in Rats.
Wanyonyi S, du Preez R, Brown L, Paul NA, Panchal SK. Wanyonyi S, et al. Nutrients. 2017 Nov 17;9(11):1261. doi: 10.3390/nu9111261. Nutrients. 2017. PMID: 29149029 Free PMC article. - Gene-diet quality interactions on haemoglobin A1c and type 2 diabetes risk: The Airwave Health Monitoring Study.
Eriksen R, Gibson R, Aresu M, Heard A, Chan Q, Evangelou E, Gao H, Elliott P, Frost G. Eriksen R, et al. Endocrinol Diabetes Metab. 2019 Jul 11;2(4):e00074. doi: 10.1002/edm2.74. eCollection 2019 Oct. Endocrinol Diabetes Metab. 2019. PMID: 31592155 Free PMC article.
References
- Gerich JE. Is reduced first-phase insulin release the earliest detectable abnormality in individuals destined to develop type 2 diabetes? Diabetes. 2002;51:S117–121. - PubMed
- Porte D. Banting lecture 1990. Beta-cells in type II diabetes mellitus. Diabetes. 1991;40:166–180. - PubMed
- Hosker JP, Rudenski AS, Burnett MA, Matthews DR, Turner RC. Similar reduction of first- and second-phase B-cell responses at three different glucose levels in type II diabetes and the effect of gliclazide therapy. Metabolism. 1989;38:767–772. - PubMed
- Hosker JP, Burnett MA, Davies EG, Harris EA, Turner RC. Sulphonylurea therapy doubles B-cell response to glucose in type 2 diabetic patients. Diabetologia. 1985;28:809–814. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical