Wnt signaling enhances neurogenesis and improves neurological function after focal ischemic injury - PubMed (original) (raw)
Wnt signaling enhances neurogenesis and improves neurological function after focal ischemic injury
Adi Shruster et al. PLoS One. 2012.
Abstract
Stroke potently stimulates cell proliferation in the subventricular zone of the lateral ventricles with subsequent neuroblast migration to the injured striatum and cortex. However, most of the cells do not survive and mature. Extracellular Wnt proteins promote adult neurogenesis in the neurogenic niches. The aim of the study was to examine the efficacy of Wnt signaling on neurogenesis and functional outcome after focal ischemic injury. Lentivirus expressing Wnt3a-HA (LV-Wnt3a-HA) or GFP (LV-GFP) was injected into the striatum or subventricular zone of mice. Five days later, focal ischemic injury was induced by injection of the vasoconstrictor endothelin-1 into the striatum of the same hemisphere. Treatment with LV-Wnt3a-HA into the striatum significantly enhanced functional recovery after ischemic injury and increased the number of BrdU-positive cells that differentiated into mature neurons in the ischemic striatum by day 28. Treatment with LV-Wnt3a-HA into the subventricular zone significantly enhanced functional recovery from the second day after injury and increased the number of immature neurons in the striatum and subventricular zone. This was accompanied by reduced dissemination of the neuronal injury. Our data indicate that Wnt signaling appears to contribute to functional recovery after ischemic injury by increasing neurogenesis or neuronal survival in the striatum.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
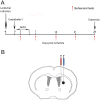
Figure 1. Schematic representation of the experimental design.
A. Experimental protocol. B. Diagram of the brain section. Lentiviral vector was injected into either the striatum (blue syringe) or SVZ (red syringe). The ischemic area in the striatum is circled in black.
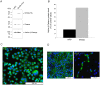
Figure 2. In vitro and in vivo validation of LV-Wnt3a-HA over-expression.
A–C. HeLa cells were infected with LV-Wnt3a-HA and confirmation of over-expression was performed using Western blot analysis (A) and immunocytochemistry (C). The ability of Wnt3a-HA to functionally activate Wnt signaling was confirmed by assessing active β-catenin (A) followed by densitometry measurements (B). Cells infected with non-related virus (NRV) were used as control. D. In vivo expression of exogenous Wnt3a-HA was detected one month after injection in the striatum.
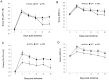
Figure 3. Effect of LV-Wnt3a-HA treatment on functional recovery.
A–B. LV-Wnt3a-HA injection into the striatum significantly improved functional performance at 28 days after injury on the cylinder test (A; p<0.05) but not on the corner test (B). C–D. LV-Wnt3a-HA treatment into the SVZ significantly improved functional recovery from day 2 after injury on the cylinder test (C; p<0.05) and on day 21 after injury on the corner test (D; p<0.05). Data are given as mean ± SEM.
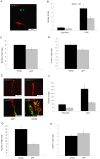
Figure 4. Effect of Wnt3a-HA treatment on neurogenesis 28 days after injury.
A. A co-localized BrdU/NeuN cell is shown in the striatum. B–D. Wnt3a-HA injection into the striatum led to a significant increase in the number of newborn neurons in the striatum (p<0.01). Treatment with Wnt3a-HA into the SVZ did not change the number of newborn neurons in the striatum. Number of newborn neurons (B), proliferating progenitors (C) and NeuN+/BrdU+ (D) cells in the striatum and SVZ.
Figure 5. Effect of LV-Wnt3a-HA injection into the SVZ on the proliferation and differentiation of progenitor cells to neuroblasts 2 days after injury.
A. EdU+ and DCX+ cells were detected in the ischemic striatum. B-D. Wnt3a-HA significantly increased the number of DCX+ cells in the striatum (p<0.01). EdU+DCX+ cells were hardly detected in the striatum. Number of newborn DCX+ (B), proliferating progenitors (C) and DCX+/EdU+ (D) cells in the striatum. E. EdU+ and DCX+ cells were found in the SVZ. D-H. Wnt3a-HA significantly increased the number of DCX+ and EdU+DCX+ cells in the SVZ (p<0.01). Number of newborn DCX+ (F), proliferating progenitors (G) and DCX+/EdU+ (H) cells in the SVZ.
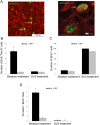
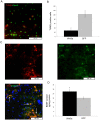
Figure 6. Neuroprotection in the ischemic striatum following LV-Wnt3a-HA injection into the SVZ 2 days after injury.
A. DNA strand breaks are labeled by TUNEL staining and NeuN immunohistochemistry in the ischemic striatum. B. Wnt3a-HA significantly reduced the number of DNA fragmented cells in the striatum (p<0.05). C. DCX+ cells manifest extensive expression of BDNF in the ischemic striatum. D. Quantification of BDNF levels in the striatum using ELISA (p<0.05).
Similar articles
- Optogenetic stimulation of glutamatergic neuronal activity in the striatum enhances neurogenesis in the subventricular zone of normal and stroke mice.
Song M, Yu SP, Mohamad O, Cao W, Wei ZZ, Gu X, Jiang MQ, Wei L. Song M, et al. Neurobiol Dis. 2017 Feb;98:9-24. doi: 10.1016/j.nbd.2016.11.005. Epub 2016 Nov 21. Neurobiol Dis. 2017. PMID: 27884724 Free PMC article. - Induction of striatal neurogenesis and generation of region-specific functional mature neurons after ischemia by growth factors. Laboratory investigation.
Yoshikawa G, Momiyama T, Oya S, Takai K, Tanaka J, Higashiyama S, Saito N, Kirino T, Kawahara N. Yoshikawa G, et al. J Neurosurg. 2010 Oct;113(4):835-50. doi: 10.3171/2010.2.JNS09989. J Neurosurg. 2010. PMID: 20345217 - Long-term administration of salvianolic acid A promotes endogenous neurogenesis in ischemic stroke rats through activating Wnt3a/GSK3β/β-catenin signaling pathway.
Zhang S, Kong DW, Ma GD, Liu CD, Yang YJ, Liu S, Jiang N, Pan ZR, Zhang W, Kong LL, Du GH. Zhang S, et al. Acta Pharmacol Sin. 2022 Sep;43(9):2212-2225. doi: 10.1038/s41401-021-00844-9. Epub 2022 Feb 25. Acta Pharmacol Sin. 2022. PMID: 35217812 Free PMC article. - Forebrain neurogenesis after focal Ischemic and traumatic brain injury.
Kernie SG, Parent JM. Kernie SG, et al. Neurobiol Dis. 2010 Feb;37(2):267-74. doi: 10.1016/j.nbd.2009.11.002. Epub 2009 Nov 10. Neurobiol Dis. 2010. PMID: 19909815 Free PMC article. Review. - Glia and Neural Stem and Progenitor Cells of the Healthy and Ischemic Brain: The Workplace for the Wnt Signaling Pathway.
Knotek T, Janeckova L, Kriska J, Korinek V, Anderova M. Knotek T, et al. Genes (Basel). 2020 Jul 16;11(7):804. doi: 10.3390/genes11070804. Genes (Basel). 2020. PMID: 32708801 Free PMC article. Review.
Cited by
- Wnt your brain be inflamed? Yes, it Wnt!
Marchetti B, Pluchino S. Marchetti B, et al. Trends Mol Med. 2013 Mar;19(3):144-56. doi: 10.1016/j.molmed.2012.12.001. Epub 2013 Jan 9. Trends Mol Med. 2013. PMID: 23312954 Free PMC article. Review. - Ischemic Preconditioning Induces Oligodendrogenesis in Mouse Brain: Effects of Nrf2 Deficiency.
Li Q, Lou J, Yang T, Wei Z, Li S, Zhang F. Li Q, et al. Cell Mol Neurobiol. 2022 Aug;42(6):1859-1873. doi: 10.1007/s10571-021-01068-5. Epub 2021 Mar 5. Cell Mol Neurobiol. 2022. PMID: 33666795 - Heme oxygenase 1-mediated neurogenesis is enhanced by Ginkgo biloba (EGb 761®) after permanent ischemic stroke in mice.
Nada SE, Tulsulkar J, Shah ZA. Nada SE, et al. Mol Neurobiol. 2014 Apr;49(2):945-56. doi: 10.1007/s12035-013-8572-x. Epub 2013 Oct 24. Mol Neurobiol. 2014. PMID: 24154866 Free PMC article. - Repair and regeneration properties of Ginkgo biloba after ischemic brain injury.
Raghavan A, Shah ZA. Raghavan A, et al. Neural Regen Res. 2014 Jul 11;9(11):1104-1107. doi: 10.4103/1673-5374.135308. Neural Regen Res. 2014. PMID: 25132842 Free PMC article. No abstract available. - Therapeutic Potential of Chinese Medicine for Endogenous Neurogenesis: A Promising Candidate for Stroke Treatment.
Li L, Li X, Han R, Wu M, Ma Y, Chen Y, Zhang H, Li Y. Li L, et al. Pharmaceuticals (Basel). 2023 May 7;16(5):706. doi: 10.3390/ph16050706. Pharmaceuticals (Basel). 2023. PMID: 37242489 Free PMC article. Review.
References
- Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 2002;8:963–970. - PubMed
- Leker RR, Soldner F, Velasco I, Gavin DK, Androutsellis-Theotokis A, et al. Long-lasting regeneration after ischemia in the cerebral cortex. Stroke. 2007;38:153–161. - PubMed
- Inestrosa N, Arenas E. Emerging roles of Wnts in the adult nervous system. Nat Rev Neurosci. 2010;11:77–86. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources