Pak1 regulates the orientation of apical polarization and lumen formation by distinct pathways - PubMed (original) (raw)
Pak1 regulates the orientation of apical polarization and lumen formation by distinct pathways
Orlando deLeon et al. PLoS One. 2012.
Abstract
The development of the basic architecture of branching tubules enclosing a central lumen that characterizes most epithelial organs crucially depends on the apico-basolateral polarization of epithelial cells. Signals from the extracellular matrix control the orientation of the apical surface, so that it faces the lumen interior, opposite to cell-matrix adhesion sites. This orientation of the apical surface is thought to be intrinsically linked to the formation of single lumens. We previously demonstrated in three-dimensional cyst cultures of Madin-Darby canine kidney (MDCK) cells that signaling by β1 integrins regulates the orientation of the apical surface, via a mechanism that depends on the activity of the small GTPase Rac1. Here, we investigated whether the Rac1 effector Pak1 is a downstream effector in this pathway. Expression of constitutive active Pak1 phenocopies the effect of β1 integrin inhibition in that it misorients the apical surface and induces a multilumen phenotype. The misorientation of apical surfaces depends on the interaction of active Pak1 with PIX proteins and is linked to defects in basement membrane assembly. In contrast, the multilumen phenotype was independent of PIX and the basement membrane. Therefore, Pak1 likely regulates apical polarization and lumen formation by two distinct pathways.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
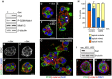
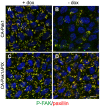
Figure 1. Constitutive active Pak1 misorients the apical surface and inhibits single lumen formation in 3D culture.
MDCK cells inducibly expressing myc-tagged CA-Pak1 under control of the Tet-off system were grown with (control, +dox) or without dox (CA-Pak1 expression, -dox). A: Lysates from four day old cells plated at confluent densities on Transwell filters were analyzed by Western blot and show expression of CA-Pak1 (myc), phospho-S298-Mek1, total Mek1/2. β-tubulin is the loading control. B–C’: Control (B,B’) and CA-Pak1-expressing cells (C,C’) were grown for four days to cyst or spheroids in 3D collagen I culture and stained for PKCζ (white in B,C, red in B’,C’) and PCX (green in B’,C’). D–G: show control (D,F) or CA-Pak1-expressing cells (E,G) grown in 3D collagen in the absence (D,E) or presence (F, G) of the β1 integrin function blocking antibody AIIB2. Cells were stained for PCX (green) and β-catenin (red). Small apical lumens in CA-Pak1-expressing cells are indicated by arrowheads. H: Quantification of phenotypes at conditions shown in D–G. n = 3. A typical image of apical inside (orange) is shown in figure D, apical outside (yellow) is shown in F, and apical mixed (blue) in E and G. I–K: Parental MDCK cells were transiently transfected with a scrambled siRNA control (con) or siRNA’s targeting Pak1 (Pak1-KD1, KD2). I: Western blot showing Pak1 knockdown by two different siRNA’s. J,K show cysts stained for PCX (green) and β-catenin (red). Nuclei in B’–G, J–K are blue, scale bars are 10 µm.
Figure 2. β1 integrin localization, synthesis and transport in Pak1-L107F expressing cells.
A–B’: Control (A) or CA-Pak1-expressing cells (B,B’) were grown in 3D collagen for 4 days and stained for F-actin (red) and β1-integrins (green). Scale bar is 10 µm. C: Control (+dox) and CA-Pak1-expressing cells (-dox) were grown on Transwell filters for 6 days and extracellular proteins were removed by mild trypsinisation. Western blots show untreated, total (PBS) and intracellular (trypsin) levels of β1 integrin as determined by a trypsin protection assay. D: Control (+dox) and CA-Pak1-expressing cells (-dox) were grown on Transwell filters for 6 days. Levels of apical (Ap) and basolateral (Bl) β1 integrin were determined by cell surface biotinylation, followed by Western blotting as shown in top panel (IP). Arrowhead shows mature β1 integrin. Levels of β1 integrin in total lysates (TL) are shown in bottom panel.
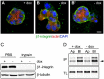
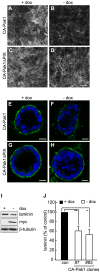
Figure 3. PIX binding is required for CA-Pak1-induced defects in apical orientation, but not lumen formation.
A: Western blot showing expression levels of different clones of CA-Pak1 and CA-Pak1ΔPIX cells grown with or without dox. Lysates were from 6-day old confluent cultures. B: Endogenous βPIX co-immunoprecipitates with CA-Pak1 but not CA-Pak1ΔPIX. Ectopically-expressed active Pak1 mutants (-dox) were immunoprecipitated with anti-myc monoclonal antibodies and associated β-PIX was detected by Western blotting with anti-β-PIX. Immunoprecipitations using anti-mouse IgG were used as negative controls. Total lysate (TL). C–D: Control cells (C, +dox) or cells expressing CA-Pak1ΔPIX (D, -dox) were grown in 3D collagen I for 4 days. Cells were stained for PCX (green), β-catenin (red) and nuclei (blue). Scale bar is 10 µm. E: Quantification of orientation of apical surfaces in spheroids described in C and D was determined as described in the legends of Figure 1H. Data show means ± SEM. n = 3. F: Quantification of spheroids with single (green) or multiple (grey) lumens. Data show means ± SEM. n = 3.
Figure 4. Constitutive active Pak1 promotes cell invasion in 3D collagen culture in a PIX-dependent manner.
A–C: Control cells (A), or cells expressing CA-Pak1 (B) or CA-Pak1ΔPIX (C) were plated in a 3D collagen I matrix and were imaged for 42–53 h. Images were taken every 11 minute, and are shown as Movies S1, S2, S3. Images show time-lapse intervals of 259 min (control), 158 (CA-Pak1) or 192 min (CA-Pak1ΔPIX) of the spheroids 29–35 h after plating. Arrowheads in CA-Pak1 cells (B) show cells that detach from the main spheroid body and invade into the collagen. Arrows in CA-Pak1ΔPIX (C) cells show transient filapodia-like protrusions. Scale bars are 50 µm.
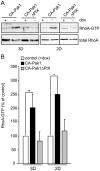
Figure 5. Activation of RhoA in 3D and 2D culture.
A: Cells were grown in the absence or presence of dox for 24 h prior to plating in a collagen I matrix (3D) or as confluent monolayers (2D), and grown for an additional two days. Western blots show levels of RhoA.GTP and total RhoA. B: Quantification of RhoA.GTP levels of cells expressing CA-Pak1 (black bars) or CA-Pak1ΔPIX (grey bars) in 2D and 3D as percentage of control (+dox, white bars). Data represent mean ± SEM. n = 3 (3D) or n = 4 (2D), **p<0.01, *p<0.02.
Figure 6. Dissolution of focal adhesions by CA-Pak1.
A–D: Confluent control cells (A,C, +dox) and cells expressing CA-Pak1 (B) or CA-Pak1ΔPIX (D) were plated on glass coverslips and stained for P-Y397-FAK (green), paxillin (red) and nuclei (blue). Images show confocal images from the basal surface and nuclei, which locate above this focal plane are therefore only partially visible. Scale bar is 10 µm.
Figure 7. CA-Pak1 inhibits laminin deposition.
A–D: Controls (+ dox, A,C) and cells expressing CA-Pak1 (B) or CA-Pak1ΔPIX (D) were grown on Transwell filters for 6 days. Cells were fixed and stained for laminin underneath the basal surface. E–H: Controls (+ dox, E,G) and cells expressing CA-Pak1 (F) or CA-Pak1ΔPIX (H) were grown in 3D collagen I for 5 days. Cells were fixed and stained for laminin (green). Nuclei, stained with DAPI, are blue. Scale bars in A–H are 10 µm. I, Western blot of total laminin levels of 2D lysates from control (+dox) and CA-Pak1 expressing cells (-dox). Myc shows expressing levels of CA-Pak1, β-tubulin is loading control. J, Quantification of total laminin in two different Pak-L107F clones. Data represent mean ± SEM. n = 3 for #7 and n = 5 for #83. **p<0.02, *p<0.05.
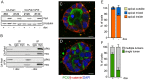
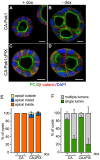
Figure 8. Endogenous matrix or BME rescues the orientation of polarization but not single lumen formation.
A–F: Cells expressing CA-Pak1 (A,B) or CA-Pak1ΔPIX (C,D) were grown in BME for four days before staining for PCX (green in A–D), β-catenin (red in A–D) and nuclei (blue in A–D). Scale bars are 10 µm. E–F: Quantification of apical orientation (F) and lumen formation (G) of cysts shown in A–D. Data show means ± SEM. n = 3.
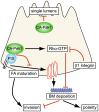
Figure 9. Model for distinct roles of Pak1 in epithelial morphogenesis.
Bottom left to right: CA-Pak1 is recruited by PIX to immature focal complexes and promotes their turnover, which inhibits cell motility and migration in a collagen I matrix and may inhibit the assembly of a basement membrane. CA-Pak1 also stimulates RhoA activation in a PIX-dependent manner, and this could inhibit basement membrane formation by a mechanism similar to what is seen in cells where β1-integrin function is inhibited . The combined effects of CA-Pak1 on migration and the basement membrane could inhibit apico-basolateral polarization and promote invasion. Top: CA-Pak1 inhibits the formation of single lumens by a process that does not depend on its interactions with PIX.
Similar articles
- A molecular switch for the orientation of epithelial cell polarization.
Bryant DM, Roignot J, Datta A, Overeem AW, Kim M, Yu W, Peng X, Eastburn DJ, Ewald AJ, Werb Z, Mostov KE. Bryant DM, et al. Dev Cell. 2014 Oct 27;31(2):171-87. doi: 10.1016/j.devcel.2014.08.027. Epub 2014 Oct 9. Dev Cell. 2014. PMID: 25307480 Free PMC article. - Pak1 regulates branching morphogenesis in 3D MDCK cell culture by a PIX and beta1-integrin-dependent mechanism.
Hunter MP, Zegers MM. Hunter MP, et al. Am J Physiol Cell Physiol. 2010 Jul;299(1):C21-32. doi: 10.1152/ajpcell.00543.2009. Epub 2010 Mar 24. Am J Physiol Cell Physiol. 2010. PMID: 20457839 Free PMC article. - The scaffold protein IQGAP1 promotes reorientation of epithelial cell polarity at the two-cell stage for cystogenesis.
Horikawa M, Hayase J, Kamakura S, Kohda A, Nakamura M, Sumimoto H. Horikawa M, et al. Genes Cells. 2024 Dec;29(12):1154-1172. doi: 10.1111/gtc.13169. Epub 2024 Oct 8. Genes Cells. 2024. PMID: 39377417 Free PMC article. - Apico-basal polarity in polycystic kidney disease epithelia.
Wilson PD. Wilson PD. Biochim Biophys Acta. 2011 Oct;1812(10):1239-48. doi: 10.1016/j.bbadis.2011.05.008. Epub 2011 Jun 1. Biochim Biophys Acta. 2011. PMID: 21658447 Review. - Mechanisms controlling human endothelial lumen formation and tube assembly in three-dimensional extracellular matrices.
Davis GE, Koh W, Stratman AN. Davis GE, et al. Birth Defects Res C Embryo Today. 2007 Dec;81(4):270-85. doi: 10.1002/bdrc.20107. Birth Defects Res C Embryo Today. 2007. PMID: 18228260 Review.
Cited by
- Integrins in Cardiovascular Health and Disease: Molecular Mechanisms and Therapeutic Opportunities.
Ławkowska K, Bonowicz K, Jerka D, Bai Y, Gagat M. Ławkowska K, et al. Biomolecules. 2025 Feb 6;15(2):233. doi: 10.3390/biom15020233. Biomolecules. 2025. PMID: 40001536 Free PMC article. Review. - The APC tumor suppressor is required for epithelial cell polarization and three-dimensional morphogenesis.
Lesko AC, Goss KH, Yang FF, Schwertner A, Hulur I, Onel K, Prosperi JR. Lesko AC, et al. Biochim Biophys Acta. 2015 Mar;1853(3):711-23. doi: 10.1016/j.bbamcr.2014.12.036. Epub 2015 Jan 8. Biochim Biophys Acta. 2015. PMID: 25578398 Free PMC article. - Evaluation of ERG responsive proteome in prostate cancer.
Tan SH, Furusato B, Fang X, He F, Mohamed AA, Griner NB, Sood K, Saxena S, Katta S, Young D, Chen Y, Sreenath T, Petrovics G, Dobi A, McLeod DG, Sesterhenn IA, Saxena S, Srivastava S. Tan SH, et al. Prostate. 2014 Jan;74(1):70-89. doi: 10.1002/pros.22731. Epub 2013 Sep 21. Prostate. 2014. PMID: 24115221 Free PMC article. - Biophysical Control of Bile Duct Epithelial Morphogenesis in Natural and Synthetic Scaffolds.
Funfak A, Bouzhir L, Gontran E, Minier N, Dupuis-Williams P, Gobaa S. Funfak A, et al. Front Bioeng Biotechnol. 2019 Dec 13;7:417. doi: 10.3389/fbioe.2019.00417. eCollection 2019. Front Bioeng Biotechnol. 2019. PMID: 31921820 Free PMC article. - Rac1 promotes kidney collecting duct integrity by limiting actomyosin activity.
Bock F, Elias BC, Dong X, Parekh DV, Mernaugh G, Viquez OM, Hassan A, Amara VR, Liu J, Brown KL, Terker AS, Chiusa M, Gewin LS, Fogo AB, Brakebusch CH, Pozzi A, Zent R. Bock F, et al. J Cell Biol. 2021 Nov 1;220(11):e202103080. doi: 10.1083/jcb.202103080. Epub 2021 Oct 14. J Cell Biol. 2021. PMID: 34647970 Free PMC article.
References
- Vega-Salas DE, Salas PJ, Gundersen D, Rodriguez-Boulan E. Formation of the apical pole of epithelial (Madin-Darby canine kidney) cells: polarity of an apical protein is independent of tight junctions while segregation of a basolateral marker requires cell-cell interactions. Journal of Cell Biology. 1987;104:905–916. - PMC - PubMed
- Lee M, Vasioukhin V. Cell polarity and cancer–cell and tissue polarity as a non-canonical tumor suppressor. J Cell Sci. 2008;121:1141–1150. - PubMed
- Lubarsky B, Krasnow MA. Tube morphogenesis: making and shaping biological tubes. Cell. 2003;112:19–28. - PubMed
- Schluter MA, Margolis B. Apical lumen formation in renal epithelia. J Am Soc Nephrol. 2009;20:1444–1452. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous