The dynamic regulation of NAD metabolism in mitochondria - PubMed (original) (raw)
The dynamic regulation of NAD metabolism in mitochondria
Liana Roberts Stein et al. Trends Endocrinol Metab. 2012 Sep.
Abstract
Mitochondria are intracellular powerhouses that produce ATP and carry out diverse functions for cellular energy metabolism. Although the maintenance of an optimal NAD/NADH ratio is essential for mitochondrial function, it has recently become apparent that the maintenance of the mitochondrial NAD pool is also of crucial importance. The biosynthesis, transport, and catabolism of NAD and its key intermediates play an important role in the regulation of NAD-consuming mediators, such as sirtuins, poly-ADP-ribose polymerases, and CD38/157 ectoenzymes, in intra- and extracellular compartments. Mitochondrial NAD biosynthesis is also modulated in response to nutritional and environmental stimuli. In this article, we discuss this dynamic regulation of NAD metabolism in mitochondria to shed light on the intimate connection between NAD and mitochondrial function.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures
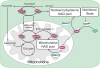
Figure 1. Maintenance of the mitochondrial NAD pool
While separate, the mitochondrial and nuclear/cytoplasmic NAD pools are intricately connected through the NAD/NADH-redox shuttles (most commonly the malate-aspartate and the glycerol-3-phosphate shuttles) and NAD biosynthetic pathways in each subcellular compartment. Multiple cellular processes play an important role in maintaining an optimal NAD/NADH ratio between mitochondria and the cytoplasm, including glycolysis, the tricarboxylic acid (TCA) cycle, and oxidative phosphorylation by the electron transport chain (ETC). Mitochondrial and nuclear/cytoplasmic NAD biosynthetic pathways are balanced in response to nutritional and environmental stimuli. Abbreviations: NAM, nicotinamide; NMN, nicotinamide mononucleotide; NAD, nicotinamide adenine dinucleotide.
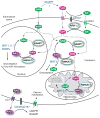
Figure 2. The biosynthesis, transport, and catabolism of NAD and its intermediates in intra- and extracellular compartments
In mammals, NAD can be synthesized from tryptophan, nicotinamide, and nicotinic acid. Nicotinamide (NAM) is a major substrate for mammalian NAD biosynthesis (see Box 1). Thus, NAD biosynthetic pathways from tryptophan and nicotinic acid are not shown in this scheme. In the cytoplasm, NAM is converted to nicotinamide mononucleotide (NMN), a key NAD intermediate, by nicotinamide phosphoribosyltransferase (NAMPT). This process might also be mediated by extracellular NAMPT (eNAMPT). It remains unclear whether a similar process occurs in the nucleus and mitochondria. Nicotinamide ribose (NR), another key NAD intermediate, can be produced from NMN extracellularly, likely by the ectoenzyme CD73, and transported into the cell, possibly through equilibrative nucleoside transporters (ENTs). Inside the cell, NR is re-phosphorylated to NMN by NR kinases 1 and 2 (NRK1 and 2). In each subcellular compartment, NMN is converted to NAD by NMN adenylyltransferases, NMNAT1-3. NMN also appears to be transported from the cytoplasm to mitochondria, although the mechanism of this NMN transport is currently unknown. NAD is consumed intracellularly by key mediators, particularly sirtuins and poly-ADP-ribose polymerases (PARPs). NAD can also be transported to the outside of the cell, likely through connexin 43 hemichannels [97]. CD38/157 ectoenzymes produce cyclic ADP-ribose (cADPR) and nicotinic acid adenosine dinucleotide phosphate (NAADP), which re-enter the cell and induce calcium mobilization.
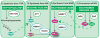
Figure I. Pathways of NAD biosynthesis
Mammalian NAD biosynthesis can occur from de novo synthesis from tryptophan (TRP) as well as conversion of the vitamin B3 precursors nicotinamide (NAM) or nicotinic acid (NA) or nicotinamide riboside (NR). These four pathways generate common downstream intermediates, as denoted by the dotted green arrows. Usage of each pathway varies in different tissues/organs. See box 1 for details.
Similar articles
- Adipose tissue NAD+-homeostasis, sirtuins and poly(ADP-ribose) polymerases -important players in mitochondrial metabolism and metabolic health.
Jokinen R, Pirnes-Karhu S, Pietiläinen KH, Pirinen E. Jokinen R, et al. Redox Biol. 2017 Aug;12:246-263. doi: 10.1016/j.redox.2017.02.011. Epub 2017 Feb 27. Redox Biol. 2017. PMID: 28279944 Free PMC article. Review. - NAD Precursors, Mitochondria Targeting Compounds and ADP-Ribosylation Inhibitors in Treatment of Inflammatory Diseases and Cancer.
Poltronieri P, Mezzolla V, Farooqi AA, Di Girolamo M. Poltronieri P, et al. Curr Med Chem. 2021;28(41):8453-8479. doi: 10.2174/0929867328666210118152653. Curr Med Chem. 2021. PMID: 33461448 Review. - NAD+ and sirtuins in aging and disease.
Imai S, Guarente L. Imai S, et al. Trends Cell Biol. 2014 Aug;24(8):464-71. doi: 10.1016/j.tcb.2014.04.002. Epub 2014 Apr 29. Trends Cell Biol. 2014. PMID: 24786309 Free PMC article. Review. - NAD and ADP-ribose metabolism in mitochondria.
Dölle C, Rack JG, Ziegler M. Dölle C, et al. FEBS J. 2013 Aug;280(15):3530-41. doi: 10.1111/febs.12304. Epub 2013 Jun 3. FEBS J. 2013. PMID: 23617329 Review. - Biosensor reveals multiple sources for mitochondrial NAD⁺.
Cambronne XA, Stewart ML, Kim D, Jones-Brunette AM, Morgan RK, Farrens DL, Cohen MS, Goodman RH. Cambronne XA, et al. Science. 2016 Jun 17;352(6292):1474-7. doi: 10.1126/science.aad5168. Science. 2016. PMID: 27313049 Free PMC article.
Cited by
- Substrate Channeling via a Transient Protein-Protein Complex: The case of D-Glyceraldehyde-3-Phosphate Dehydrogenase and L-Lactate Dehydrogenase.
Svedružić ŽM, Odorčić I, Chang CH, Svedružić D. Svedružić ŽM, et al. Sci Rep. 2020 Jun 26;10(1):10404. doi: 10.1038/s41598-020-67079-2. Sci Rep. 2020. PMID: 32591631 Free PMC article. - Improvement of gamete quality by stimulating and feeding the endogenous antioxidant system: mechanisms, clinical results, insights on gene-environment interactions and the role of diet.
Dattilo M, D'Amato G, Caroppo E, Ménézo Y. Dattilo M, et al. J Assist Reprod Genet. 2016 Dec;33(12):1633-1648. doi: 10.1007/s10815-016-0767-4. Epub 2016 Jul 16. J Assist Reprod Genet. 2016. PMID: 27423667 Free PMC article. Review. - Regulation of Glucose Metabolism by NAD+ and ADP-Ribosylation.
Hopp AK, Grüter P, Hottiger MO. Hopp AK, et al. Cells. 2019 Aug 13;8(8):890. doi: 10.3390/cells8080890. Cells. 2019. PMID: 31412683 Free PMC article. Review. - Impact of Fenugreek on Milk Production in Rodent Models of Lactation Challenge.
Sevrin T, Alexandre-Gouabau MC, Castellano B, Aguesse A, Ouguerram K, Ngyuen P, Darmaun D, Boquien CY. Sevrin T, et al. Nutrients. 2019 Oct 24;11(11):2571. doi: 10.3390/nu11112571. Nutrients. 2019. PMID: 31653107 Free PMC article. - Reversibility of Age-related Oxidized Free NADH Redox States in Alzheimer's Disease Neurons by Imposed External Cys/CySS Redox Shifts.
Dong Y, Sameni S, Digman MA, Brewer GJ. Dong Y, et al. Sci Rep. 2019 Aug 2;9(1):11274. doi: 10.1038/s41598-019-47582-x. Sci Rep. 2019. PMID: 31375701 Free PMC article.
References
- Szendroedi J, et al. The role of mitochondria in insulin resistance and type 2 diabetes mellitus. Nat Rev Endocrinol. 2011;8:92–103. - PubMed
- Solaini G, et al. Oxidative phosphorylation in cancer cells. Biochim Biophys Acta. 2011;1807:534–542. - PubMed
- Kristian T, et al. Mitochondrial dysfunction and nicotinamide dinucleotide catabolism as mechanisms of cell death and promising targets for neuroprotection. J Neurosci Res. 2011;89:1946–1955. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 5T32GM007067/GM/NIGMS NIH HHS/United States
- R01 AG037457/AG/NIA NIH HHS/United States
- R01 AG024150/AG/NIA NIH HHS/United States
- T32 GM007067/GM/NIGMS NIH HHS/United States
- P30 DK056341/DK/NIDDK NIH HHS/United States
- P30DK056341/DK/NIDDK NIH HHS/United States
- AG024150/AG/NIA NIH HHS/United States
- P60DK202579/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials