Redefinition of the carbohydrate binding specificity of Helicobacter pylori BabA adhesin - PubMed (original) (raw)
Redefinition of the carbohydrate binding specificity of Helicobacter pylori BabA adhesin
John Benktander et al. J Biol Chem. 2012.
Abstract
Certain Helicobacter pylori strains adhere to the human gastric epithelium using the blood group antigen-binding adhesin (BabA). All BabA-expressing H. pylori strains bind to the blood group O determinants on type 1 core chains, i.e. to the Lewis b antigen (Fucα2Galβ3(Fucα4)GlcNAc; Le(b)) and the H type 1 determinant (Fucα2Galβ3GlcNAc). Recently, BabA strains have been categorized into those recognizing only Le(b) and H type 1 determinants (designated specialist strains) and those that also bind to A and B type 1 determinants (designated generalist strains). Here, the structural requirements for carbohydrate recognition by generalist and specialist BabA were further explored by binding of these types of strains to a panel of different glycosphingolipids. Three glycosphingolipids recognized by both specialist and generalist BabA were isolated from the small intestine of a blood group O pig and characterized by mass spectrometry and proton NMR as H type 1 pentaglycosylceramide (Fucα2Galβ3GlcNAcβ3Galβ4Glcβ1Cer), Globo H hexaglycosylceramide (Fucα2Galβ3GalNAcβ3Galα4Galβ4Glcβ1Cer), and a mixture of three complex glycosphingolipids (Fucα2Galβ4GlcNAcβ6(Fucα2Galβ3GlcNAcβ3)Galβ3GlcNAcβ3Galβ4Glcβ1Cer, Fucα2Galβ3GlcNAcβ6(Fucα2Galβ3GlcNAcβ3)Galβ3GlcNAcβ3Galβ4Glcβ1Cer, and Fucα2Galβ4(Fucα3)GlcNAcβ6(Fucα2Galβ3GlcNAcβ3)Galβ3GlcNAcβ3Galβ4Glcβ1Cer). In addition to the binding of both strains to the Globo H hexaglycosylceramide, i.e. a blood group O determinant on a type 4 core chain, the generalist strain bound to the Globo A heptaglycosylceramide (GalNAcα3(Fucα2)Galβ3GalNAcβ3Galα4Galβ4Glcβ1Cer), i.e. a blood group A determinant on a type 4 core chain. The binding of BabA to the two sets of isoreceptors is due to conformational similarities of the terminal disaccharides of H type 1 and Globo H and of the terminal trisaccharides of A type 1 and Globo A.
Figures
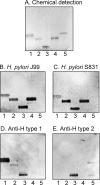
FIGURE 1.
Binding of a generalist and a specialist H. pylori strain to non-acid glycosphingolipids of the small intestinal epithelium of a blood group O pig. The glycosphingolipids were separated on aluminum-backed silica gel plates using chloroform/methanol/water (60:35:8 by volume) as the solvent system. The chromatogram in A was stained with anisaldehyde. Duplicate chromatograms were incubated with the 35S-labeled H. pylori generalist strain J99 (B) and the H. pylori specialist strain S831 (C) followed by autoradiography for 12 h as described under “Experimental Procedures.” Lane 1, non-acid glycosphingolipids of the intestinal epithelium of a blood group O pig, 40 μg; lane 2, reference H type 2 pentaglycosylceramide (Fucα2Galβ4GlcNAcβ3Galβ4Glcβ1Cer), 4 μg; lane 3, reference Lea pentaglycosylceramide (Galβ3(Fucα4)GlcNAcβ3Galβ4Glcβ1Cer), 2 μg; lane 4, reference Leb hexaglycosylceramide (Fucα2Galβ3(Fucα4)GlcNAcβ3Galβ4Glcβ1Cer), 4 μg; lane 5, reference B type 1 heptaglycosylceramide (Galα3(Fucα2)Galβ3(Fucα4)GlcNAcβ3Galβ4Glcβ1Cer), 2 μg.
FIGURE 2.
_H. pylori_-binding glycosphingolipids isolated from the small intestinal epithelium of a blood group O pig. The glycosphingolipids were separated on aluminum-backed silica gel plates using chloroform/methanol/water (60:35:8 by volume) as the solvent system. The chromatogram in A was stained with anisaldehyde. Duplicate chromatograms were incubated with the 35S-labeled H. pylori generalist strain J99 (B), the H. pylori specialist strain S831 (C), the monoclonal anti-H type 1 antibody 17-206 (D), and the monoclonal anti-H type 2 antibody 92FR-A2 (E) followed by autoradiography for 12 h as described under “Experimental Procedures.” Lane 1, fraction P-I isolated from pig intestine, 2 μg; lane 2, fraction P-II from pig intestine, 2 μg; lane 3, fraction P-III from pig intestine, 2 μg; lane 4, reference Leb hexaglycosylceramide (Fucα2Galβ3(Fucα4)GlcNAcβ3Galβ4Glcβ1Cer), 2 μg; lane 5, reference H type 2 pentaglycosylceramide (Fucα2Galβ4GlcNAcβ3Galβ4Glcβ1Cer), 2 μg.
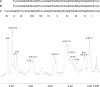
FIGURE 3.
Characterization of the H. pylori BabA-binding fraction P-II from the small intestinal epithelium of a blood group O pig. A, base peak chromatogram from LC-ESI/MS of the oligosaccharides obtained by digestion of the H. pylori BabA-binding fraction P-II with Rhodococcus endoglycoceramidase II. B, mass chromatogram of m/z 852. C, mass chromatogram of m/z 1014. D, MS2 spectrum of the [M − H+]− ion at m/z 852 (retention time (RT), 26.8 min). The interpretation formula shows the deduced oligosaccharide sequence. E, MS2 spectrum of the [M − H+]− ion at m/z 1014 (retention time, 25.3 min). The interpretation formula shows the deduced oligosaccharide sequence. F, anomeric region of the 600-MHz proton NMR spectrum of fraction P-II (30 °C). The sample was dissolved in dimethyl sulfoxide/D2O (98:2 by volume) after deuterium exchange.
FIGURE 4.
LC-ESI/MS of the decasaccharides obtained by hydrolysis of _H. pylori_-binding fraction P-III with Rhodococcus endoglycoceramidase II. A, MS2 spectrum of the [M − 2H+]2− ion at m/z 864 (retention time (RT), 23.4 min). B, MS2 spectrum of the [M − 2H+]2− ion at m/z 864 (retention time, 26.3 min). C, MS3 spectrum of the ion at m/z 1201 (retention time, 23.4 min). D, MS3 spectrum of the ion at m/z 1201 (retention time, 26.3 min). E, interpretation formula showing the deduced oligosaccharide sequence.
FIGURE 5.
LC-ESI/MS of the undecasaccharide obtained by hydrolysis of _H. pylori_-binding fraction P-III with Rhodococcus endoglycoceramidase II. A, MS2 spectrum of the [M − 2H+]2− ion at m/z 937 (retention time (RT), 25.1 min). B, MS3 spectrum of the ion at m/z 1712 (retention time, 25.1 min). C, interpretation formula showing the deduced oligosaccharide sequence.
FIGURE 6.
Anomeric region of the 600-MHz proton NMR spectrum of the _H. pylori_-binding fraction P-III from porcine small intestinal epithelium (30 °C). The sample was dissolved in dimethyl sulfoxide/D2O (98:2 by volume) after deuterium exchange.
FIGURE 7.
Comparison of binding of a generalist and a specialist H. pylori strain to reference glycosphingolipids. The glycosphingolipids were separated on aluminum-backed silica gel plates using chloroform/methanol/water (60:35:8 by volume) as the solvent system. The chromatogram in A was stained with anisaldehyde. Duplicate chromatograms were incubated with the 35S-labeled H. pylori generalist strain J99 (B) and the H. pylori specialist strain S831 (C) followed by autoradiography for 12 h as described under “Experimental Procedures.” Lane 1, Leb hexaglycosylceramide (Fucα2Galβ3(Fucα4)GlcNAcβ3Galβ4Glcβ1Cer), 2 μg; lane 2, B type 1 heptaglycosylceramide (Galα3(Fucα2)Galβ3(Fucα4)GlcNAcβ3Galβ4Glcβ1Cer), 2 μg; lane 3, A type 1 heptaglycosylceramide (GalNAcα3(Fucα2)Galβ3(Fucα4)GlcNAcβ3Galβ4Glcβ1Cer), 2 μg; lane 4, A type 1 hexaglycosylceramide (GalNAcα3(Fucα2)Galβ3GlcNAcβ3Galβ4Glcβ1Cer), 2 μg; lane 5, Globo A/A type 4 heptaglycosylceramide (GalNAcα3(Fucα2)Galβ3GalNAcβ3Galα4Galβ4Glcβ1Cer), 2 μg; lane 6, A nonaglycosylceramide (GalNAcα3(Fucα2)Galβ3GalNAcα3(Fucα2)Galβ3GlcNAcβ3Galβ4Glcβ1Cer), 2 μg; lane 7, A type 2 heptaglycosylceramide (GalNAcα3(Fucα2)Galβ4(Fucα3)GlcNAcβ3Galβ4Glcβ1Cer), 2 μg.
FIGURE 8.
Comparison of binding of a generalist and a specialist H. pylori strain to dilutions of pure glycosphingolipids on thin-layer chromatograms. The glycosphingolipids were separated on aluminum-backed silica gel plates using chloroform/methanol/water (60:35:8 by volume) as the solvent system. The chromatograms were incubated with the 35S-labeled H. pylori generalist strain J99 (A) and the H. pylori specialist strain S831 (B) followed by autoradiography for 12 h as described under “Experimental Procedures.” Lane 1, Leb hexaglycosylceramide (Leb-6; Fucα2Galβ3(Fucα4)GlcNAcβ3Galβ4Glcβ1Cer), 200 ng, and A nonaglycosylceramide (A9-1; GalNAcα3(Fucα2)Galβ3GalNAcα3(Fucα2)Galβ3GlcNAcβ3Galβ4Glcβ1Cer), 200 ng; lane 2, Leb hexaglycosylceramide, 80 ng, and A nonaglycosylceramide, 80 ng; lane 3, Leb hexaglycosylceramide, 40 ng, and A nonaglycosylceramide, 40 ng; lane 4, Globo A/A type 4 heptaglycosylceramide (Globo A; GalNAcα3(Fucα2)Galβ3GalNAcβ3Galα4Galβ4Glcβ1Cer), 200 ng; lane 5, Globo A/A type 4 heptaglycosylceramide, 80 ng; lane 6, Globo A/A type 4 heptaglycosylceramide, 40 ng; lane 7, A type 1 heptaglycosylceramide (A7-1; GalNAcα3(Fucα2)Galβ3(Fucα4)GlcNAcβ3Galβ4Glcβ1Cer), 200 ng; lane 8, A type 1 heptaglycosylceramide, 80 ng; lane 9, A type 1 heptaglycosylceramide, 40 ng.
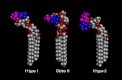
FIGURE 9.
Minimum energy models of the H type 1 pentaglycosylceramide (Fucα2Galβ3GlcNAcβ3Galβ4Glcβ1Cer) (left), the Globo H hexaglycosylceramide (Fucα2Galβ3GalNAcβ3Galα4Galβ4Glcβ1Cer) (center), and the H type 2 pentaglycosylceramide (Fucα2Galβ4GlcNAcβ3Galβ4Glcβ1Cer) (right). The terminal disaccharides are colored blue (Fucα2) and purple (Galβ3/4). The terminal part of the BabA-binding H type 1 and Globo H glycosphingolipids can be aligned by assuming different Glcβ1Cer torsion angles, whereas the H type 2 terminal disaccharide is rotated ∼90° in comparison, rendering this glycosphingolipid non-binding with respect to BabA.
FIGURE 10.
Binding of monoclonal antibodies to human gastric glycosphingolipids. Thin-layer chromatogram after detection with anisaldehyde (A) and autoradiograms obtained by binding of the monoclonal anti-Globo H antibody MBr1 (B), the monoclonal anti-Leb antibody BG-6/T218 (C), and the monoclonal anti-H type 1 antibody 17-206 (D) are shown. The chromatograms were eluted with chloroform/methanol/water (60:35:8 by volume), and the binding assays were done as described under “Experimental Procedures” followed by autoradiography for 12 h. Lane 1, non-acid glycosphingolipids of human stomach (Individual I; blood group O), 40 μg; lane 2, non-acid glycosphingolipids of human stomach (Individual II; blood group A), 40 μg; lane 3, reference Leb hexaglycosylceramide (Fucα2Galβ3(Fucα4)GlcNAcβ3Galβ4Glcβ1Cer), 2 μg; lane 4, non-acid glycosphingolipids of the intestinal epithelium of a blood group O pig, 40 μg; lane 5, non-acid glycosphingolipids of the intestinal epithelium of a blood group A pig, 40 μg; lane 6, reference H type 1 pentaglycosylceramide (Fucα2Galβ3GlcNAcβ3Galβ4Glcβ1Cer), 2 μg; lane 7, reference Globo H hexaglycosylceramide (Fucα2Galβ3GalNAcβ3Galα4Galβ4Glcβ1Cer), 1 μg; lane 8, reference Globo A heptaglycosylceramide (GalNAcα3(Fucα2)Galβ3GalNAcβ3Galα4Galβ4Glcβ1Cer), 4 μg.
Similar articles
- Recognition of blood group ABH type 1 determinants by the FedF adhesin of F18-fimbriated Escherichia coli.
Coddens A, Diswall M, Angström J, Breimer ME, Goddeeris B, Cox E, Teneberg S. Coddens A, et al. J Biol Chem. 2009 Apr 10;284(15):9713-26. doi: 10.1074/jbc.M807866200. Epub 2009 Feb 10. J Biol Chem. 2009. PMID: 19208633 Free PMC article. - _Helicobacter pylori_-binding nonacid glycosphingolipids in the human stomach.
Jin C, Barone A, Borén T, Teneberg S. Jin C, et al. J Biol Chem. 2018 Nov 2;293(44):17248-17266. doi: 10.1074/jbc.RA118.004854. Epub 2018 Sep 19. J Biol Chem. 2018. PMID: 30232154 Free PMC article. - Dynamics of Lewis b binding and sequence variation of the babA adhesin gene during chronic Helicobacter pylori infection in humans.
Nell S, Kennemann L, Schwarz S, Josenhans C, Suerbaum S. Nell S, et al. mBio. 2014 Dec 16;5(6):e02281-14. doi: 10.1128/mBio.02281-14. mBio. 2014. PMID: 25516619 Free PMC article. - Roles of Helicobacter pylori BabA in gastroduodenal pathogenesis.
Yamaoka Y. Yamaoka Y. World J Gastroenterol. 2008 Jul 21;14(27):4265-72. doi: 10.3748/wjg.14.4265. World J Gastroenterol. 2008. PMID: 18666312 Free PMC article. Review. - Helicobacter pylori BabA in adaptation for gastric colonization.
Ansari S, Yamaoka Y. Ansari S, et al. World J Gastroenterol. 2017 Jun 21;23(23):4158-4169. doi: 10.3748/wjg.v23.i23.4158. World J Gastroenterol. 2017. PMID: 28694656 Free PMC article. Review.
Cited by
- Adhesion of Helicobacter Species to the Human Gastric Mucosa: A Deep Look Into Glycans Role.
Matos R, Amorim I, Magalhães A, Haesebrouck F, Gärtner F, Reis CA. Matos R, et al. Front Mol Biosci. 2021 May 7;8:656439. doi: 10.3389/fmolb.2021.656439. eCollection 2021. Front Mol Biosci. 2021. PMID: 34026832 Free PMC article. Review. - Helicobacter pylori Outer Membrane Protein-Related Pathogenesis.
Matsuo Y, Kido Y, Yamaoka Y. Matsuo Y, et al. Toxins (Basel). 2017 Mar 11;9(3):101. doi: 10.3390/toxins9030101. Toxins (Basel). 2017. PMID: 28287480 Free PMC article. Review. - Characterization of the rainbow trout (Oncorhynchus mykiss) mucosal glycosphingolipid repertoire and Aeromonas salmonicida binding to neutral glycosphingolipids.
Benktander J, Sundh H, Sundell K, Sharba S, Teneberg S, Lindén SK. Benktander J, et al. Glycobiology. 2024 Jul 26;34(9):cwae055. doi: 10.1093/glycob/cwae055. Glycobiology. 2024. PMID: 39107988 Free PMC article. - The Role of Helicobacter pylori Outer Membrane Proteins in Adherence and Pathogenesis.
Oleastro M, Ménard A. Oleastro M, et al. Biology (Basel). 2013 Aug 27;2(3):1110-34. doi: 10.3390/biology2031110. Biology (Basel). 2013. PMID: 24833057 Free PMC article. - Helicobacter pylori Virulence Factors-Mechanisms of Bacterial Pathogenicity in the Gastric Microenvironment.
Baj J, Forma A, Sitarz M, Portincasa P, Garruti G, Krasowska D, Maciejewski R. Baj J, et al. Cells. 2020 Dec 25;10(1):27. doi: 10.3390/cells10010027. Cells. 2020. PMID: 33375694 Free PMC article. Review.
References
- Karlsson K. A. (1989) Animal glycosphingolipids as membrane attachment sites for bacteria. Annu. Rev. Biochem. 58, 309–350 - PubMed
- Esko J. D. (1999) Essentials in Glycobiology (Varki A., Cummings R., Esko J., Freeze H., Hart G., Marth J., eds) pp. 429–440, Cold Spring Harbor Laboratory, Cold Spring Harbor, NY - PubMed
- Pieters R. J. (2011) Carbohydrate mediated bacterial adhesion. Adv. Exp. Med. Biol. 715, 227–240 - PubMed
- Stults C. L., Sweeley C. C., Macher B. A. (1989) Glycosphingolipids: structure, biological source and properties. Methods Enzymol. 179, 167–214 - PubMed
- Borén T., Falk P., Roth K. A., Larson G., Normark S. (1993) Attachment of Helicobacter pylori to human gastric epithelium mediated by blood group antigens. Science 262, 1892–1895 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources