The chemokine receptor cxcr5 regulates the regenerative neurogenesis response in the adult zebrafish brain - PubMed (original) (raw)
The chemokine receptor cxcr5 regulates the regenerative neurogenesis response in the adult zebrafish brain
Caghan Kizil et al. Neural Dev. 2012.
Abstract
Background: Unlike mammals, zebrafish exhibits extensive neural regeneration after injury in adult stages of its lifetime due to the neurogenic activity of the radial glial cells. However, the genes involved in the regenerative neurogenesis response of the zebrafish brain are largely unknown. Thus, understanding the underlying principles of this regeneration capacity of the zebrafish brain is an interesting research realm that may offer vast clinical ramifications.
Results: In this paper, we characterized the expression pattern of cxcr5 and analyzed the function of this gene during adult neurogenesis and regeneration of the zebrafish telencephalon. We found that cxcr5 was upregulated transiently in the RGCs and neurons, and the expression in the immune cells such as leukocytes was negligible during both adult neurogenesis and regeneration. We observed that the transgenic misexpression of cxcr5 in the ventricular cells using dominant negative and full-length variants of the gene resulted in altered proliferation and neurogenesis response of the RGCs. When we knocked down cxcr5 using antisense morpholinos and cerebroventricular microinjection, we observed outcomes similar to the overexpression of the dominant negative cxcr5 variant.
Conclusions: Thus, based on our results, we propose that cxcr5 imposes a proliferative permissiveness to the radial glial cells and is required for differentiation of the RGCs to neurons, highlighting novel roles of cxcr5 in the nervous system of vertebrates. We therefore suggest that cxcr5 is an important cue for ventricular cell proliferation and regenerative neurogenesis in the adult zebrafish telencephalon. Further studies on the role of cxcr5 in mediating neuronal replenishment have the potential to produce clinical ramifications in efforts for regenerative therapeutic applications for human neurological disorders or acute injuries.
Figures
Figure 1
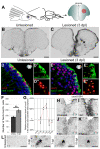
cxcr5 is expressed in radial glial cells (RGCs) and neurons in the adult zebrafish telencephalon. (A) Schematic representation of an adult zebrafish telencephalon. A stab lesion is performed in one hemisphere (red circle on the cross section scheme). (B)cxcr5 is expressed along the ventricular region in the unlesioned telencephalon. (C)cxcr5 expression after a lesion (asterisk) is stronger in the lesioned hemisphere along the ventricular region. (D)cxcr5 fluorescent in situ hybridization (FISH) coupled to green fluorescent protein (GFP) immunohistochemistry in Tg(her4.1:GFP) transgenics in unlesioned the adult zebrafish telencephalon; counterstained with 4,6-diamidino-2-phenylindole (DAPI). (D’) Individual channel for her4.1:GFP. (D”) Individual channel for cxcr5. Radial glial cells (white asterisks) and periventricular cells (yellow asterisks) express cxcr5. (E)cxcr5 FISH coupled to GFP staining in Tg(her4.1:GFP) transgenics in the 3 day post-lesion adult zebrafish telencephalon; counterstained with DAPI. (E’) Individual channel for her4.1:GFP. (E”) Individual channel for cxcr5. Radial glial cells (white asterisks) and periventricular cells (yellow asterisks) express cxcr5. Note the number of _cxcr5_-positive periventricular cells increased in comparison to the unlesioned region. (F) Graph indicating the number of her4-cxcr5 double-positive cells before and after inducing the lesion. (G) Quantitative real-time PCR analysis for cxcr5 expression at different time points after the lesion. (H-K) Time-course cxcr5 in situ hybridization analyses on the unlesioned region (H), 3 dpl (I), 7 dpl (J) and 15 dpl (K) telencephalons. (L)cxcr5 expression around the lesion site. Lesion site is denoted by an asterisk; n ≥ 3 telencephalons for every analysis. Scale bars 50 μm (B, C, H-L), and 10 μm (D-E”).
Figure 2
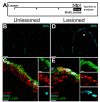
cxcr5 is expressed in proliferating radial glial cells (RGCs). (A) Scheme for experimental setup. At 3 days after a lesion or a sham operation, bromo-deoxyuridine (BrdU) is applied for 6 hours before sacrificing the animals. (B) BrdU immunohistochemistry on the unlesioned (sham-operated) adult zebrafish telencephalon section showing the proliferating cells. (C)cxcr5 fluorescent in situ hybridization (FISH) coupled to BrdU and GFP immunohistochemistry on unlesioned Tg(her4.1:GFP) transgenics. Insets show single channel images. BrdU-positive RGCs express cxcr5 (white asterisk). (D) BrdU immunohistochemistry on the 3 days post-lesion (dpl) adult zebrafish telencephalon section. Asterisk indicates the lesion site. (E)cxcr5 FISH coupled to BrdU and GFP immunohistochemistry on 3 dpl Tg(her4.1:GFP) transgenics. Insets show single channel images. BrdU-positive RGCs increase in number and they express cxcr5 (white asterisks). Scale bars 50 μm in B and D, and 10 μm in C and E; n = 3 telencephalons.
Figure 3
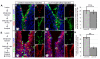
cxcr5 is sufficient to increase ventricular cell proliferation after injury in the adult zebrafish telencephalon. (A) Cxcr5 is a seven-span transmembrane protein with an extracellular receptor domain, seven transmembrane domains and a C-terminus intracellular domain. We generated full-length and dominant-negative versions of Cxcr5. Both variants are inserted into a transgenesis cassette containing the enhanced green fluorescent protein (EGFP) reporter and self-cleaving T2A. The whole cassette is expressed under heat-inducible hsp70l promoter. (B) Heat shock scheme. Four heat shocks, three of which are after the lesion or sham operation, were given before sacrifice and analysis. (C) Proliferating cell nuclear antigen (PCNA) and green fluorescent protein (GFP) immunohistochemistry (IHC) in telencephalons of unlesioned (sham-operated) non-transgenic animals. C’ and C” are PCNA and GFP. (D) PCNA and GFP IHC in telencephalons of unlesioned (sham-operated) Tg(hsp:egfp-t2a-dncxcr5) animals. D’ and D” are PCNA and GFP. (E) PCNA and GFP IHC in telencephalons of unlesioned (sham-operated) Tg(hsp:egfp-t2a-FLcxcr5) animals. E’ and E” are PCNA and GFP. (F) Quantification graph for the relative amounts of GFP and PCNA double-positive cells in unlesioned telencephalons, where the transgenic misexpression of cxcr5 does not effect cell proliferation. (G) PCNA and GFP IHC in telencephalons of 3 days post-lesion (dpl) non-transgenic animals. G’ and G” are PCNA and GFP. (H) PCNA and GFP IHC in telencephalons of 3 dpl Tg(hsp:egfp-t2a-dncxcr5) animals. H’ and H” are PCNA and GFP. (I) PCNA and GFP IHC in telencephalons of 3 dpl Tg(hsp:egfp-t2a-FLcxcr5) animals. I’ and I” are PCNA and GFP. (J) Quantification graph for the relative amounts of GFP and PCNA double-positive in 3 dpl telencephalons, where transgenic misexpression of the full-length cxcr5 increases but the dominant negative variant does not affect the ventricular cell proliferation. Scale bars 25 μm; n = 4 telencephalons for each set of analyses.
Figure 4
cxcr5 is required and sufficient for regenerative neurogenesis. (A) Heat shock and BrdU scheme for neurogenesis assay in the unlesioned telencephalon. After a sham operation, three daily heat shocks were given before a 10-hour BrdU pulse and sacrifice at 30 days after the sham operation. (B-D) HuC and BrdU immunohistochemistry (IHC) and 4,6-diamidino-2-phenylindole (DAPI) counterstaining on unlesioned telencephalons from non-transgenic (B), Tg(hsp:egfp-t2a-dncxcr5)(C) and Tg(hsp:egfp-t2a-FLcxcr5)(D) animals. Primed and double-primed images are single channels for BrdU and HuC. (E) Quantification graph for relative numbers of HuC and BrdU double positive cells (newborn neurons) in unlesioned telencephalons. Transgenic misexpression of cxcr5 does not alter the constitutive levels of neurogenesis in the adult zebrafish telencephalon. (F) Heat shock and BrdU scheme for neurogenesis assay in lesioned telencephalons. After the lesion, three daily heat shocks were given before a 10-hour BrdU pulse and sacrifice at 30 days after lesioning. (G-I) HuC and BrdU IHC and DAPI counterstaining on unlesioned telencephalons from non-transgenic (G), Tg(hsp:egfp-t2a-dncxcr5)(H) and Tg(hsp:egfp-t2a-FLcxcr5)(I) animals. Primed and double-primed images are single channels for BrdU and HuC. (J) Quantification graph for relative numbers of newborn neurons in lesioned telencephalons. Transgenic misexpression of dominant negative variant of cxcr5 significantly reduces, and full-length cxcr5 significantly increases, the number of newborn neurons after lesioning in the adult zebrafish telencephalon. Scale bars 25 μm; n = 4 telencephalons for each analysis.
Figure 5
Knocking down cxcr5 with cerebroventricular microinjection (CVMI) results in reduced regenerative neurogenesis but not radial glial cell proliferation. (A) Lesion, CVMI and BrdU treatment scheme. (B) BrdU and S100β immunohistochemistry (IHC) with 4,6-diamidino-2-phenylindole (DAPI) counterstaining on control morpholino-injected brains. (B’) BrdU channel. (B”) S100β channel. (C) BrdU and S100β IHC with DAPI counterstaining on cxcr5 antisense morpholino-injected brains. (C’) BrdU channel. (C”) S100β channel. (D) Graph showing the average number of proliferating glial cells. Knocking down cxcr5 does not alter the levels of proliferating radial glial cells. (E) Lesion, CVMI, BrdU treatment and pulse-chase scheme. (F) BrdU and HuC IHC with DAPI counterstaining on control morpholino-injected brains. (F’) BrdU channel. (F”) HuC channel. (G) BrdU and HuC IHC with DAPI counterstaining on cxcr5 antisense morpholino-injected brains. (G’) BrdU channel. (G”) HuC channel. (H) Graph showing the average number of newborn neurons. Knocking down cxcr5 significantly reduces the levels of regenerative neurogenesis.
Figure 6
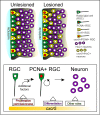
Summary and working model for cxcr5 expression and function in unlesioned and lesioned adult zebrafish telencephalons. In unlesioned adult zebrafish telencephalons, cxcr5 is expressed in proliferating and non-proliferating radial glial cells (RGCs), as well as neurons in the periventricular region. After a lesion, proliferating _cxcr5_-positive RGCs and _cxcr5_-positive periventricular neurons increase in number. cxcr5 expression is strongest at the ventricular surface and weakens towards the deeper layers of parenchyma. Based on the transgenic misexpression of the dominant negative and the full-length variants of cxcr5 gene in the ventricular zone cells, we propose that cxcr5 is a permissive cue for proliferative competency of the RGCs. cxcr5 is sufficient to enhance proliferation of RGCs not before but after injury, possibly due to additional injury-induced factors required in combination with cxcr5 to enhance cell proliferation. Based on the expression of cxcr5 in differentiating neurons and long-term neurogenesis experiments upon transgenic misexpression, we suggest that cxcr5 is also required for differentiation of RGCs to neurons. Since cxcr5 is also expressed in neurons, it might have other roles in neurons, such as migration, which are yet to be identified.
Similar articles
- Neuron-glia interaction through Serotonin-BDNF-NGFR axis enables regenerative neurogenesis in Alzheimer's model of adult zebrafish brain.
Bhattarai P, Cosacak MI, Mashkaryan V, Demir S, Popova SD, Govindarajan N, Brandt K, Zhang Y, Chang W, Ampatzis K, Kizil C. Bhattarai P, et al. PLoS Biol. 2020 Jan 6;18(1):e3000585. doi: 10.1371/journal.pbio.3000585. eCollection 2020 Jan. PLoS Biol. 2020. PMID: 31905199 Free PMC article. - Regenerative neurogenesis from neural progenitor cells requires injury-induced expression of Gata3.
Kizil C, Kyritsis N, Dudczig S, Kroehne V, Freudenreich D, Kaslin J, Brand M. Kizil C, et al. Dev Cell. 2012 Dec 11;23(6):1230-7. doi: 10.1016/j.devcel.2012.10.014. Epub 2012 Nov 15. Dev Cell. 2012. PMID: 23168169 - Increased radial glia quiescence, decreased reactivation upon injury and unaltered neuroblast behavior underlie decreased neurogenesis in the aging zebrafish telencephalon.
Edelmann K, Glashauser L, Sprungala S, Hesl B, Fritschle M, Ninkovic J, Godinho L, Chapouton P. Edelmann K, et al. J Comp Neurol. 2013 Sep 1;521(13):3099-115. doi: 10.1002/cne.23347. J Comp Neurol. 2013. PMID: 23787922 - Zebrafish as a translational regeneration model to study the activation of neural stem cells and role of their environment.
Ceci M, Mariano V, Romano N. Ceci M, et al. Rev Neurosci. 2018 Dec 19;30(1):45-66. doi: 10.1515/revneuro-2018-0020. Rev Neurosci. 2018. PMID: 30067512 Review. - Adult neurogenesis and brain regeneration in zebrafish.
Kizil C, Kaslin J, Kroehne V, Brand M. Kizil C, et al. Dev Neurobiol. 2012 Mar;72(3):429-61. doi: 10.1002/dneu.20918. Dev Neurobiol. 2012. PMID: 21595047 Review.
Cited by
- Autoimmune-Mediated Retinopathy in CXCR5-Deficient Mice as the Result of Age-Related Macular Degeneration Associated Proteins Accumulation.
Lennikov A, Saddala MS, Mukwaya A, Tang S, Huang H. Lennikov A, et al. Front Immunol. 2019 Aug 14;10:1903. doi: 10.3389/fimmu.2019.01903. eCollection 2019. Front Immunol. 2019. PMID: 31474986 Free PMC article. - Regeneration of the central nervous system-principles from brain regeneration in adult zebrafish.
Zambusi A, Ninkovic J. Zambusi A, et al. World J Stem Cells. 2020 Jan 26;12(1):8-24. doi: 10.4252/wjsc.v12.i1.8. World J Stem Cells. 2020. PMID: 32110272 Free PMC article. Review. - Regeneration, Plasticity, and Induced Molecular Programs in Adult Zebrafish Brain.
Cosacak MI, Papadimitriou C, Kizil C. Cosacak MI, et al. Biomed Res Int. 2015;2015:769763. doi: 10.1155/2015/769763. Epub 2015 Aug 31. Biomed Res Int. 2015. PMID: 26417601 Free PMC article. Review. - Comparative aspects of adult neural stem cell activity in vertebrates.
Grandel H, Brand M. Grandel H, et al. Dev Genes Evol. 2013 Mar;223(1-2):131-47. doi: 10.1007/s00427-012-0425-5. Epub 2012 Nov 22. Dev Genes Evol. 2013. PMID: 23179636 Review. - Neuron-glia interaction through Serotonin-BDNF-NGFR axis enables regenerative neurogenesis in Alzheimer's model of adult zebrafish brain.
Bhattarai P, Cosacak MI, Mashkaryan V, Demir S, Popova SD, Govindarajan N, Brandt K, Zhang Y, Chang W, Ampatzis K, Kizil C. Bhattarai P, et al. PLoS Biol. 2020 Jan 6;18(1):e3000585. doi: 10.1371/journal.pbio.3000585. eCollection 2020 Jan. PLoS Biol. 2020. PMID: 31905199 Free PMC article.
References
- Chapouton P, Jagasia R, Bally-Cuif L. Adult neurogenesis in non-mammalian vertebrates. Bioessays. 2007. pp. 745–757. - PubMed
- Baumgart EV, Barbosa JS, Bally-Cuif L, Gotz M, Ninkovic J. Stab wound injury of the zebrafish telencephalon: A model for comparative analysis of reactive gliosis. Glia. 2011;60:343–357. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases