Brain dendritic cells: biology and pathology - PubMed (original) (raw)
Review
Brain dendritic cells: biology and pathology
Paul M D'Agostino et al. Acta Neuropathol. 2012 Nov.
Abstract
Dendritic cells (DC) are the professional antigen-presenting cells of the immune system. In their quiescent and mature form, the presentation of self-antigens by DC leads to tolerance; whereas, antigen presentation by mature DC, after stimulation by pathogen-associated molecular patterns, leads to the onset of antigen-specific immunity. DC have been found in many of the major organs in mammals (e.g. skin, heart, lungs, intestines and spleen); while the brain has long been considered devoid of DC in the absence of neuroinflammation. Consequently, microglia, the resident immune cell of the brain, have been charged with many functional attributes commonly ascribed to DC. Recent evidence has challenged the notion that DC are either absent or minimal players in brain immune surveillance. This review will discuss the recent literature examining DC involvement within both the young and aged steady-state brain. We will also examine DC contributions during various forms of neuroinflammation resulting from neurodegenerative autoimmune disease, injury, and CNS infections. This review also touches upon DC trafficking between the central nervous system and peripheral immune compartments during viral infections, the new molecular technologies that could be employed to enhance our current understanding of brain DC ontogeny, and some potential therapeutic uses of DC within the CNS.
Conflict of interest statement
Conflict of interest The authors have no conflicting financial interests.
Figures
Fig. 1
Putative DC within the brain can interact with CD4+ T lymphocytes following viral-induced neuroinflammation. Representative confocal Z-stack analysis of a VSV intranasally infected olfactory bulb at 4 (left) and 7 (right) days post-infection. CD11c/EYFP+ cells (green) are found throughout glomerular tissue richly populated with CD4+ T cells (red). Inset depicts a confocal section in which CD11c/EYFP+ cells are in physical contact with CD4+ T cells. Representative images from three experiments with an n =3; scale bar 50 μm (10 μm inset)
Fig. 2
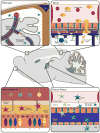
Schematic representation of tissue reservoirs believed to be the source of DC infiltration during neuroinflammation. In the steady-state brain Flt3+ DC are primarily found within the meninges and choroid plexus. Evidence for DC-like cells has also been found within circumventricular organs such as the area postrema, subfornical organ, median eminence, and pituitary (not depicted for simplicity). During neuroinflammation (e.g., of viral etiology) the DC found within these area are postulated to enter the brain parenchyma in response to cytokine/chemokine gradients. Other potential sources of infiltrating DC are the nasal mucosa, nasal-associated lymphoid tissue, and the blood, in the form of monocyte-derived DC (moDC). Therefore, in light of these various reservoirs, the methods of DC infiltration are believed to range from direct migration into brain tissue, diapedesis followed by crossing of the glia limitans from the Virchow–Robin spaces, and passage from CSF through the CVOs into the brain parenchyma; image not to scale
Similar articles
- Brain dendritic cells and macrophages/microglia in central nervous system inflammation.
Fischer HG, Reichmann G. Fischer HG, et al. J Immunol. 2001 Feb 15;166(4):2717-26. doi: 10.4049/jimmunol.166.4.2717. J Immunol. 2001. PMID: 11160337 - Dendritic cells as therapeutic targets in neuroinflammation.
Luessi F, Zipp F, Witsch E. Luessi F, et al. Cell Mol Life Sci. 2016 Jul;73(13):2425-50. doi: 10.1007/s00018-016-2170-9. Epub 2016 Mar 12. Cell Mol Life Sci. 2016. PMID: 26970979 Free PMC article. Review. - CCR2-dependent dendritic cell accumulation in the central nervous system during early effector experimental autoimmune encephalomyelitis is essential for effector T cell restimulation in situ and disease progression.
Clarkson BD, Walker A, Harris MG, Rayasam A, Sandor M, Fabry Z. Clarkson BD, et al. J Immunol. 2015 Jan 15;194(2):531-41. doi: 10.4049/jimmunol.1401320. Epub 2014 Dec 10. J Immunol. 2015. PMID: 25505278 Free PMC article. - Immune heterogeneity in neuroinflammation: dendritic cells in the brain.
Colton CA. Colton CA. J Neuroimmune Pharmacol. 2013 Mar;8(1):145-62. doi: 10.1007/s11481-012-9414-8. Epub 2012 Nov 1. J Neuroimmune Pharmacol. 2013. PMID: 23114889 Free PMC article. Review. - The role of dendritic cells in neurodegenerative diseases.
Iribarren P, Cui YH, Le Y, Wang JM. Iribarren P, et al. Arch Immunol Ther Exp (Warsz). 2002;50(3):187-96. Arch Immunol Ther Exp (Warsz). 2002. PMID: 12098934 Review.
Cited by
- More than microglia: myeloid cells and biomarkers in neurodegeneration.
Kodosaki E, Bell R, Sogorb-Esteve A, Wiltshire K, Zetterberg H, Heslegrave A. Kodosaki E, et al. Front Neurosci. 2024 Oct 31;18:1499458. doi: 10.3389/fnins.2024.1499458. eCollection 2024. Front Neurosci. 2024. PMID: 39544911 Free PMC article. Review. - Biosensor-Enhanced Organ-on-a-Chip Models for Investigating Glioblastoma Tumor Microenvironment Dynamics.
Thenuwara G, Javed B, Singh B, Tian F. Thenuwara G, et al. Sensors (Basel). 2024 Apr 30;24(9):2865. doi: 10.3390/s24092865. Sensors (Basel). 2024. PMID: 38732975 Free PMC article. Review. - Exploring dendritic cell subtypes in cancer immunotherapy: unraveling the role of mature regulatory dendritic cells.
Badillo O, Helfridsson L, Niemi J, Hellström M. Badillo O, et al. Ups J Med Sci. 2024 Apr 12;129. doi: 10.48101/ujms.v129.10627. eCollection 2024. Ups J Med Sci. 2024. PMID: 38716077 Free PMC article. Review. - Potential roles for efferocytosis in glioblastoma immune evasion.
Lorimer IAJ. Lorimer IAJ. Neurooncol Adv. 2024 Jan 25;6(1):vdae012. doi: 10.1093/noajnl/vdae012. eCollection 2024 Jan-Dec. Neurooncol Adv. 2024. PMID: 38616895 Free PMC article. - Glioblastoma patients' survival and its relevant risk factors during the pre-COVID-19 and post-COVID-19 pandemic: real-world cohort study in the USA and China.
Qin L, Li H, Zheng D, Lin S, Ren X. Qin L, et al. Int J Surg. 2024 May 1;110(5):2939-2949. doi: 10.1097/JS9.0000000000001224. Int J Surg. 2024. PMID: 38376848 Free PMC article.
References
- Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood–brain barrier. Nat Rev Neurosci. 2006;7(1):41–53. - PubMed
- Agger R, Crowley MT, Witmer-Pack MD. The surface of dendritic cells in the mouse as studied with monoclonal antibodies. Int Rev Immunol. 1990;6(2–3):89–101. - PubMed
- Aleyas AG, George JA, Han YW, Rahman MM, Kim SJ, Han SB, Kim BS, Kim K, Eo SK. Functional modulation of dendritic cells and macrophages by Japanese encephalitis virus through MyD88 adaptor molecule-dependent and -independent pathways. J Immunol. 2009;183(4):2462–2474. - PubMed
- Ali S, Curtin JF, Zirger JM, Xiong W, King GD, Barcia C, Liu C, Puntel M, Goverdhana S, Lowenstein PR, Castro MG. Inflammatory and anti-glioma effects of an adenovirus expressing human soluble Fms-like tyrosine kinase 3 ligand (hsFlt3L): treatment with hsFlt3L inhibits intracranial glioma progression. Mol Ther. 2004;10(6):1071–1084. - PMC - PubMed
- Anandasabapathy N, Victora GD, Meredith M, Feder R, Dong B, Kluger C, Yao K, Dustin ML, Nussenzweig MC, Steinman RM, Liu K. Flt3L controls the development of radiosensitive dendritic cells in the meninges and choroid plexus of the steady-state mouse brain. J Exp Med. 2011;208(8):1695–1705. - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources