Macrophage migration inhibitory factor mediates the antidepressant actions of voluntary exercise - PubMed (original) (raw)
. 2012 Aug 7;109(32):13094-9.
doi: 10.1073/pnas.1205535109. Epub 2012 Jul 23.
Se Hyun Kim, Yong Ryoul Yang, Parkyong Song, Hyun Sook Yu, Hong Geun Park, Onyou Hwang, Whaseon Lee-Kwon, Jeong Kon Seo, Daehee Hwang, Jang Hyun Choi, Richard Bucala, Sung Ho Ryu, Yong Sik Kim, Pann-Ghill Suh
Affiliations
- PMID: 22826223
- PMCID: PMC3420190
- DOI: 10.1073/pnas.1205535109
Macrophage migration inhibitory factor mediates the antidepressant actions of voluntary exercise
Hyo Youl Moon et al. Proc Natl Acad Sci U S A. 2012.
Abstract
Voluntary exercise is known to have an antidepressant effect. However, the underlying mechanism for this antidepressant action of exercise remains unclear, and little progress has been made in identifying genes that are directly involved. We have identified macrophage migration inhibitory factor (MIF) by analyzing existing mRNA microarray data and confirmed the augmented expression of selected genes under two experimental conditions: voluntary exercise and electroconvulsive seizure. A proinflammatory cytokine, MIF is expressed in the central nervous system and involved in innate and adaptive immune responses. A recent study reported that MIF is involved in antidepressant-induced hippocampal neurogenesis, but the mechanism remains elusive. In our data, tryptophan hydroxylase 2 (Tph2) and brain-derived neurotrophic factor (Bdnf) expression were induced after MIF treatment in vitro, as well as during both exercise and electroconvulsive seizure in vivo. This increment of Tph2 was accompanied by increases in the levels of total serotonin in vitro. Moreover, the MIF receptor CD74 and the ERK1/2 pathway mediate the MIF-induced Tph2 and Bdnf gene expression as well as serotonin content. Experiments in Mif(-/-) mice revealed depression-like behaviors and a blunted antidepressant effect of exercise, as reflected by changes in Tph2 and Bdnf expression in the forced swim test. In addition, administration of recombinant MIF protein produced antidepressant-like behavior in rats in the forced swim test. Taken together, these results suggest a role of MIF in mediating the antidepressant action of exercise, probably by enhancing serotonin neurotransmission and neurotrophic factor-induced neurogenesis in the brain.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
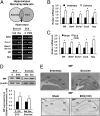
Fig. 1.
MIF is induced by long-term voluntary exercise and repeated ECS treatments in rat hippocampus. (A) Scheme and RT-PCR results for selecting candidate genes regulated by both voluntary exercise and ECS. (B and C) Real-time RT-PCR was performed to measure the mRNA expression of five genes among candidate genes in rat hippocampi. mRNA expression was enhanced in long-term exercise-treated rats (28 d; n = 8) and after repeated ECS treatments (10 d; n = 5). (D) Quantitative immunoblotting showed that both exercise and ECS increased the level of MIF protein in the rat hippocampi (n = 4). (E) Immunohistochemistry showing that exercise (28 d) and ECS (10 d) increased MIF immunostaining in the rat hippocampus. Sed, sedentary; Exe, exercise; S, sham. Error bars show mean ± SE. Asterisks indicate statistically significant differences compared with the control groups (P < 0.05).
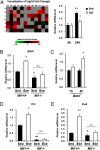
Fig. 2.
MIF increases expression of Bdnf in vitro and in vivo. (A) (Left) Heat maps representing the relative expression levels for all genes (involved in neurogenesis and neuronal stem cells) induced by treatment with MIF (300 ng/mL) for 24 h on the real-time PCR arrays. Data for each gene were normalized to a panel of housekeeping transcripts and are expressed as fold change compared with the vehicle-treated group. (Right) Real-time RT-PCR was performed to measure mRNA expression of the selected MIF-target genes from RT-PCR and the PubMed search, Bdnf and Fgf2. Treatment with recombinant MIF protein (300 ng/mL) for 24 h increased the expression level of Bdnf and Fgf2. Error bars show mean ± SD. *P < 0.05; **P < 0.01 vs. 0 h. (B) Bdnf mRNA expression was reduced in the hippocampi of _Mif_−/−mice compared with WT littermates. Furthermore, Bdnf mRNA expression level was not significantly changed by long-term exercise in hippocampi of _Mif_−/−mice compared with sedentary _Mif_−/−mice (n = 6–8 for each group). Error bars show mean ± SE. #P < 0.05; *P < 0.05. (C) ICV injection of MIF protein increased Bdnf gene expression at 4 h after injection (n = 4–6 for each group). (D and E) Dcx and Pax6 mRNA expression was reduced in the hippocampi of _Mif_−/− mice compared with WT littermates. In addition, the Dcx and Pax6 mRNA expression level was not changed significantly by long-term exercise in the hippocampus of _Mif_−/− mice compared with sedentary _Mif_−/− mice (n = 6–8 for each group). Error bars show mean ± SE. NS, not significant. *P < 0.05; #P < 0.05; ##P < 0.01.
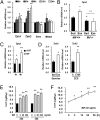
Fig. 3.
MIF activates the 5-HT system. (A) Treatment with recombinant MIF protein (300 ng/mL) increased the expression level of Tph2 in a time-dependent manner among neurotransmission-related genes. Error bars show mean ± SD. *P < 0.05 vs. 0 h. (B) Tph2 mRNA expression was reduced in the hippocampus of _Mif_−/− mice compared with WT littermates. In addition, the Tph2 mRNA expression level was not changed significantly by long-term exercise in the hippocampi of _Mif_−/− mice compared with sedentary _Mif_−/− mice (n = 6–8 for each group). Error bars show mean ± SE. *P < 0.05; #P < 0.05. (C) ICV injection of MIF protein increases Tph2 gene expression at 1 h and 4 h after injection (n = 4–6 for each group). (D) Both repeated ECS treatments and voluntary exercise increased Tph2 gene expression in the rat hippocampus (n = 4–6 for each group). (E) Treatment with MIF induced a dose-dependent (3, 30, and 300 ng/mL) increase in 5-HT levels in RBL2H3 cells at 24 h and 36 h after treatment (n = 3). (F) MIF (300 ng/mL) treatment induced a time-dependent (3, 12, 24, and 36 h) increase in 5-HT levels in RBL2H3 cells (n = 3). NS, not significant. Error bars show mean ± SD. *P < 0.05; **P < 0.01 vs. 0 h.
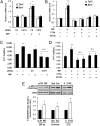
Fig. 4.
MIF increases the amount of 5-HT via the CD74-ERK1/2 pathway. (A) Down-regulation of CD74 prevented MIF action on the expression of Tph2 and Bdnf. *P < 0.05 vs. control siRNA (or control siRNA with MIF treatment). (B) Pretreatment with CT04 (5 μg/mL), a GTPase RhoA inhibitor, or U0126 (10 μM), a selective MEK inhibitor, diminished the MIF (300 ng/mL)-induced expression of Tph2 and Bdnf in Neuro-2A cells. *P < 0.05; **P < 0.01 vs. the vehicle treatment (or vehicle with MIF treatment). (C and D) Down-regulation of Cd74 reduced the basal amount of 5-HT. Cd74 siRNA transfection and pretreatment with CT04 (5 μg/mL) or U0126 (10 μM) prevented the amount of 5-HT induction at 24 h after MIF treatment (300 ng/mL). NS, not significant. Error bars show mean ± SD. *P < 0.05; #P < 0.05 vs. control siRNA (or control siRNA with MIF treatment). **P < 0.01 vs. the vehicle treatment (or vehicle with MIF treatment). (E) ICV administration of MIF, chronic exercise, and repeated ECS treatments increased ERK1/2 phosphorylation in the rat hippocampi.
Fig. 5.
Antidepressant action of MIF as evidenced by the behavioral test. (A) _Mif_−/− (KO) mice had longer immobility times compared with WT littermates, and no antidepressant action of exercise was evident in _Mif_−/− mice on the FST (n = 6–8 for each group). (B) ICV injection of recombinant MIF protein (5 μL of a 300-μg/mL solution) into rats significantly reduced immobility time on the FST at 4 h after injection (n = 8–10 for each group). IMM, immobility; SWM, swimming; CLM, climbing. Error bars show mean ± SE. *P < 0.05. (C) Schematic model showing the mechanism of MIF on the antidepressant effect induced by exercise.
Comment in
- Psychiatric disorders: Run for your MIF.
Welberg L. Welberg L. Nat Rev Neurosci. 2012 Sep;13(9):602. doi: 10.1038/nrn3328. Epub 2012 Aug 16. Nat Rev Neurosci. 2012. PMID: 22895476 No abstract available.
Similar articles
- Macrophage migration inhibitory factor (MIF) exerts antifibrotic effects in experimental liver fibrosis via CD74.
Heinrichs D, Knauel M, Offermanns C, Berres ML, Nellen A, Leng L, Schmitz P, Bucala R, Trautwein C, Weber C, Bernhagen J, Wasmuth HE. Heinrichs D, et al. Proc Natl Acad Sci U S A. 2011 Oct 18;108(42):17444-9. doi: 10.1073/pnas.1107023108. Epub 2011 Oct 3. Proc Natl Acad Sci U S A. 2011. PMID: 21969590 Free PMC article. - Macrophage migration inhibitory factor facilitates production of CCL5 in astrocytes following rat spinal cord injury.
Zhou Y, Guo W, Zhu Z, Hu Y, Wang Y, Zhang X, Wang W, Du N, Song T, Yang K, Guan Z, Wang Y, Guo A. Zhou Y, et al. J Neuroinflammation. 2018 Sep 4;15(1):253. doi: 10.1186/s12974-018-1297-z. J Neuroinflammation. 2018. PMID: 30180853 Free PMC article. - The critical role of macrophage migration inhibitory factor in insulin activity.
Vujicic M, Senerovic L, Nikolic I, Saksida T, Stosic-Grujicic S, Stojanovic I. Vujicic M, et al. Cytokine. 2014 Sep;69(1):39-46. doi: 10.1016/j.cyto.2014.05.013. Epub 2014 Jun 4. Cytokine. 2014. PMID: 25022960 - Behavioral and serotonergic consequences of decreasing or increasing hippocampus brain-derived neurotrophic factor protein levels in mice.
Deltheil T, Guiard BP, Cerdan J, David DJ, Tanaka KF, Repérant C, Guilloux JP, Coudoré F, Hen R, Gardier AM. Deltheil T, et al. Neuropharmacology. 2008 Nov;55(6):1006-14. doi: 10.1016/j.neuropharm.2008.08.001. Epub 2008 Aug 12. Neuropharmacology. 2008. PMID: 18761360 Review. - Possible role of exercise therapy on depression: Effector neurotransmitters as key players.
Alizadeh Pahlavani H. Alizadeh Pahlavani H. Behav Brain Res. 2024 Feb 29;459:114791. doi: 10.1016/j.bbr.2023.114791. Epub 2023 Dec 2. Behav Brain Res. 2024. PMID: 38048912 Review.
Cited by
- The effectiveness of mind-body therapy and physical training in alleviating depressive symptoms in adult cancer patients: a meta-analysis.
Zeng Y, Huang R, Zhao L, He X, Mao S. Zeng Y, et al. J Cancer Res Clin Oncol. 2024 Jun 5;150(6):289. doi: 10.1007/s00432-024-05813-3. J Cancer Res Clin Oncol. 2024. PMID: 38836958 Free PMC article. Review. - MIF contribution to progressive brain diseases.
Matejuk A, Benedek G, Bucala R, Matejuk S, Offner H, Vandenbark AA. Matejuk A, et al. J Neuroinflammation. 2024 Jan 4;21(1):8. doi: 10.1186/s12974-023-02993-6. J Neuroinflammation. 2024. PMID: 38178143 Free PMC article. Review. - Major Depressive Disorder and Gut Microbiota: Role of Physical Exercise.
Souza PB, de Araujo Borba L, Castro de Jesus L, Valverde AP, Gil-Mohapel J, Rodrigues ALS. Souza PB, et al. Int J Mol Sci. 2023 Nov 28;24(23):16870. doi: 10.3390/ijms242316870. Int J Mol Sci. 2023. PMID: 38069198 Free PMC article. Review. - Neurobiological effects of exercise intervention for premenstrual syndrome.
Hwang RJ, Chen HJ, Ni LF, Liu TY, Shih YL, Chuang YO. Hwang RJ, et al. Cogn Neurodyn. 2023 Oct;17(5):1297-1308. doi: 10.1007/s11571-022-09893-0. Epub 2022 Oct 15. Cogn Neurodyn. 2023. PMID: 37786666 Free PMC article. - Exercise prevents fatal stress-induced myocardial injury in obese mice.
Dun Y, Hu Z, You B, Du Y, Zeng L, Zhao Y, Liu Y, Wu S, Cui N, Yang F, Liu S. Dun Y, et al. Front Endocrinol (Lausanne). 2023 Aug 29;14:1223423. doi: 10.3389/fendo.2023.1223423. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37711889 Free PMC article.
References
- Belmaker RH, Agam G. Major depressive disorder. N Engl J Med. 2008;358:55–68. - PubMed
- Cassano P, Fava M. Tolerability issues during long-term treatment with antidepressants. Ann Clin Psychiatry. 2004;16:15–25. - PubMed
- Wong ML, Licinio J. Research and treatment approaches to depression. Nat Rev Neurosci. 2001;2:343–351. - PubMed
- Eley TC, et al. Gene-environment interaction analysis of serotonin system markers with adolescent depression. Mol Psychiatry. 2004;9:908–915. - PubMed
- Santarelli L, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous