A NAC transcription factor and SNI1 cooperatively suppress basal pathogen resistance in Arabidopsis thaliana - PubMed (original) (raw)
. 2012 Oct;40(18):9182-92.
doi: 10.1093/nar/gks683. Epub 2012 Jul 22.
Hyeong Cheol Park, Kyung Eun Kim, Mi Soon Jung, Hay Ju Han, Sun Ho Kim, Young Sang Kwon, Sunghwa Bahk, Jonguk An, Dong Won Bae, Dae-Jin Yun, Sang-Soo Kwak, Woo Sik Chung
Affiliations
- PMID: 22826500
- PMCID: PMC3467076
- DOI: 10.1093/nar/gks683
A NAC transcription factor and SNI1 cooperatively suppress basal pathogen resistance in Arabidopsis thaliana
Ho Soo Kim et al. Nucleic Acids Res. 2012 Oct.
Abstract
Transcriptional repression of pathogen defense-related genes is essential for plant growth and development. Several proteins are known to be involved in the transcriptional regulation of plant defense responses. However, mechanisms by which expression of defense-related genes are regulated by repressor proteins are poorly characterized. Here, we describe the in planta function of CBNAC, a calmodulin-regulated NAC transcriptional repressor in Arabidopsis. A T-DNA insertional mutant (cbnac1) displayed enhanced resistance to a virulent strain of the bacterial pathogen Pseudomonas syringae DC3000 (PstDC3000), whereas resistance was reduced in transgenic CBNAC overexpression lines. The observed changes in disease resistance were correlated with alterations in pathogenesis-related protein 1 (PR1) gene expression. CBNAC bound directly to the PR1 promoter. SNI1 (suppressor of nonexpressor of PR genes1, inducible 1) was identified as a CBNAC-binding protein. Basal resistance to PstDC3000 and derepression of PR1 expression was greater in the cbnac1 sni1 double mutant than in either cbnac1 or sni1 mutants. SNI1 enhanced binding of CBNAC to its cognate PR1 promoter element. CBNAC and SNI1 are hypothesized to work as repressor proteins in the cooperative suppression of plant basal defense.
Figures
Figure 1.
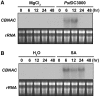
CBNAC expression is induced by pathogen- and SA. (A) Induction of CBNAC gene expression by _Pst_DC3000. Leaves of 4-week-old Arabidopsis plants (Col-0) were infiltrated with a bacterial suspension (OD600 = 0.001 in 10 mM MgCl2). Infitrated leaves were harvested at the indicated times after inoculation. The gel blot analysis of total RNA that was performed with a 32P-labeled CBNAC probe is shown. Ethidium bromide-stained rRNA is shown as loading control. (B) Induction of CBNAC gene expression by SA. Leaves of 4-week-old Arabidopsis plants (Col-0) were treated with 1 mM SA. Leaf collection, RNA isolation and RNA gel blot analysis was performed as in (A).
Figure 2.
CBNAC negatively regulates resistance to _Pst_DC3000 and PR1 expression. Leaves of wild type (WT), 35S:Flag-CBNAC and cbnac1 plants were inoculated with a bacterial suspension (OD600 = 0.001 in 10 mM MgCl2). (A) Growth of _Pst_DC3000 in inoculated leaves at 0 and 3 dpi. Mean bacterial densities ± SE were calculated from six to eight replicate plants are shown. Significant differences as calculated by Student’s t test (P < 0.05) are indicated by unique letters. The experiment was repeated at least three times with similar results. (B) qRT-PCR analysis of PR1 expression in inoculated leaves. Values were normalized using the expression level of Tubulin 2 and expressed relative to the expression level in WT at 12 hpi, which is arbitrarily set at 100. Mean relative expression values ± SE from three independent experiments are shown. Data were analyzed by Student’s t test. Different letters indicate statistically significant differences between genotypes (P < 0.05).
Figure 3.
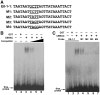
CBNAC interacts with the E0-1-1 element of the PR1 promoter. (A) Nucleotide sequence of the native and mutated (M1–M4) E0-1-1 elements used in EMSA. (B) Analysis of binding specificity. EMSA was performed using 32P-labeled native E0-1-1 as probe as above except that GST-CBNAC protein was preincubated with 50- (lane 4), 100- (lane 5) or 200- (lane 6) fold molar excess of cold native E0-1-1 (competitor) before addition of probe. (C) EMSA of CBNAC binding. 32P-labeled native (lanes 1–3) and mutated (lanes 4–7) E0-1-1 probes were incubated with equal amounts of _E. coli_-expressed GST-CBNAC (lanes 3 to 7) or GST alone (lanes 1 and 2) before electrophoresis.
Figure 4.
CBNAC interacts with SNI1. (A) Yeast two-hybrid analysis. Transformants of yeast strain pJ69-4A were grown as indicated (upper left) on minimal medium with (+Ade) or without (–Ade) selection. Adenine prototrophy indicates positive interaction. β-Galactosidase activity in the colonies grown in +Ade medium was determined by filter-lift assay (LacZ). (B) LCI assay for detecting interaction in planta. Tobacco leaves were transformed by Agrobacterium infiltration using a needleless syringe. The indicated NLuc and CLuc construct pairs were used for transformation. Shown are luminescence images (upper panel) and quantitative luminescence measurements (lower panel) depicting luciferase activity in inoculated leaves at 48 hpi.
Figure 5.
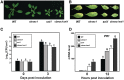
Altered responses of the cbnac1 sni1 double mutant to _Pst_DC3000. (A) Morphology of 5-week-old wild-type (WT), cbnac1, sni1 and cbnac1 sni1 plants grown on MS agar plates. (B–D) Disease resistance responses in leaves inoculated with bacterial suspension as in Figure 3. Disease symptoms in inoculated leaves at 5 dpi are depicted (B). Bacterial growth in inoculated leaves at 0 and 3 dpi are compared (C). Mean bacterial densities ± SE were calculated from six to eight replicate plants. Significant differences as calculated by Student’s t test (P < 0.05) are indicated by unique letters. The experiment was repeated at least three times with similar results. PR1 expression was monitored in inoculated leaves by qRT-PCR (D). Values were normalized using the expression level of Tubulin 2 and expressed relative to the expression level in WT at 12hpi, which is arbitrarily set at 100. Mean relative expression values ±SE values from three independent experiments are shown. Data were analyzed by Student’s t test. Different letters indicate statistically significant differences between genotypes (P < 0.05).
Figure 6.
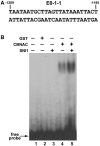
SNI1 enhances binding of CBNAC to the E0-1-1 element. (A) Nucleotide sequence of the PR1 promoter indicating E0-1-1 element. The numbers indicate the position of the element relative to the PR1 translation start site. (B) EMSA analysis of the effect of SNI1. EMSA was performed using 32P-labeled E0-1-1 element (lanes 1 to 5), without (lane 1) or with the addition GST (lane 2; negative control), CBNAC (lanes 4 and 5) and SNI1 (lane 5). Equal amounts of CBNAC were used in the two lanes.
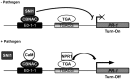
Figure 7.
Model for the regulation of PR1 by the CBNAC-SNI1 complex. In non-induced conditions (–Pathogen), because SNI1 does not contain a known DNA-binding domain, we postulate that SNI1 binds to CBNAC and is thereby recruited the E0-1-1 element of PR1 promoter. SNI1 enhances the DNA-binding activity of CBNAC and somehow this enhances repression of PR1 by SNI1. In the presence of inducer (+Pathogen), PR1 gene expression is induced by the translocation of a large amount of active NPR1 to the nucleus and its interaction with TGA transcription factors. The SNI1/CBNAC protein complex can be removed by NPR1, CaM or other unknown mechanisms.
Similar articles
- DNA repair proteins are directly involved in regulation of gene expression during plant immune response.
Song J, Durrant WE, Wang S, Yan S, Tan EH, Dong X. Song J, et al. Cell Host Microbe. 2011 Feb 17;9(2):115-24. doi: 10.1016/j.chom.2011.01.011. Cell Host Microbe. 2011. PMID: 21320694 - Overexpression of Arabidopsis ACBP3 enhances NPR1-dependent plant resistance to Pseudomonas syringe pv tomato DC3000.
Xiao S, Chye ML. Xiao S, et al. Plant Physiol. 2011 Aug;156(4):2069-81. doi: 10.1104/pp.111.176933. Epub 2011 Jun 13. Plant Physiol. 2011. PMID: 21670223 Free PMC article. - A comprehensive structure-function analysis of Arabidopsis SNI1 defines essential regions and transcriptional repressor activity.
Mosher RA, Durrant WE, Wang D, Song J, Dong X. Mosher RA, et al. Plant Cell. 2006 Jul;18(7):1750-65. doi: 10.1105/tpc.105.039677. Epub 2006 Jun 9. Plant Cell. 2006. PMID: 16766691 Free PMC article. - Ethylene Response Factor ERF11 Activates BT4 Transcription to Regulate Immunity to Pseudomonas syringae.
Zheng X, Xing J, Zhang K, Pang X, Zhao Y, Wang G, Zang J, Huang R, Dong J. Zheng X, et al. Plant Physiol. 2019 Jun;180(2):1132-1151. doi: 10.1104/pp.18.01209. Epub 2019 Mar 29. Plant Physiol. 2019. PMID: 30926656 Free PMC article. - Actin branches out to link pathogen perception and host gene regulation.
Porter K, Day B. Porter K, et al. Plant Signal Behav. 2013 Mar;8(3):e23468. doi: 10.4161/psb.23468. Epub 2013 Jan 18. Plant Signal Behav. 2013. PMID: 23333960 Free PMC article. Review.
Cited by
- Transcription factor NTL9 negatively regulates Arabidopsis vascular cambium development during stem secondary growth.
Sugimoto H, Tanaka T, Muramoto N, Kitagawa-Yogo R, Mitsukawa N. Sugimoto H, et al. Plant Physiol. 2022 Oct 27;190(3):1731-1746. doi: 10.1093/plphys/kiac368. Plant Physiol. 2022. PMID: 35951755 Free PMC article. - Regulatory Plasticity of Earthworm wMT-2 Gene Expression.
Drechsel V, Schauer K, Šrut M, Höckner M. Drechsel V, et al. Int J Mol Sci. 2017 May 24;18(6):1113. doi: 10.3390/ijms18061113. Int J Mol Sci. 2017. PMID: 28538660 Free PMC article. - GLRaV-2 protein p24 suppresses host defenses by interaction with a RAV transcription factor from grapevine.
Zhang C, Wang X, Li H, Wang J, Zeng Q, Huang W, Huang H, Xie Y, Yu S, Kan Q, Wang Q, Cheng Y. Zhang C, et al. Plant Physiol. 2022 Jun 27;189(3):1848-1865. doi: 10.1093/plphys/kiac181. Plant Physiol. 2022. PMID: 35485966 Free PMC article. - TaNAC1 acts as a negative regulator of stripe rust resistance in wheat, enhances susceptibility to Pseudomonas syringae, and promotes lateral root development in transgenic Arabidopsis thaliana.
Wang F, Lin R, Feng J, Chen W, Qiu D, Xu S. Wang F, et al. Front Plant Sci. 2015 Feb 27;6:108. doi: 10.3389/fpls.2015.00108. eCollection 2015. Front Plant Sci. 2015. PMID: 25774162 Free PMC article. - A New Species in Pseudophialophora From Wild Rice and Beneficial Potential.
Zhu JN, Yu YJ, Dai MD, Zeng YL, Lu XJ, Wang L, Liu XH, Su ZZ, Lin FC. Zhu JN, et al. Front Microbiol. 2022 Mar 11;13:845104. doi: 10.3389/fmicb.2022.845104. eCollection 2022. Front Microbiol. 2022. PMID: 35359723 Free PMC article.
References
- Dangl JL, Jones JD. Plant pathogens and integrated defence responses to infection. Nature. 2001;411:826–833. - PubMed
- Durrant WE, Dong X. Systemic acquired resistance. Annu. Rev. Phytopathol. 2004;42:185–209. - PubMed
- Glazebrook J. Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annu. Rev. Phytopathol. 2005;43:205–227. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials