Persistent neuroinflammatory effects of serial exposure to stress and methamphetamine on the blood-brain barrier - PubMed (original) (raw)
Persistent neuroinflammatory effects of serial exposure to stress and methamphetamine on the blood-brain barrier
Nicole A Northrop et al. J Neuroimmune Pharmacol. 2012 Dec.
Abstract
Studies of methamphetamine (Meth)-induced neurotoxicity have traditionally focused on monoaminergic terminal damage while more recent studies have found that stress exacerbates these damaging effects of Meth. Similarities that exist between the mechanisms that cause monoaminergic terminal damage in response to stress and Meth and those capable of producing a disruption of the blood-brain barrier (BBB) suggest that the well-known high co-morbidity of stress and Meth could produce long-lasting structural and functional BBB disruption. The current studies examined the role of neuroinflammation in mediating the effects of exposure to chronic stress and/or Meth on BBB structure and function. Rats were pre-exposed to chronic unpredictable stress (CUS) and/or challenged with Meth. Twenty-four hours after the treatment of Meth in rats pre-exposed to CUS, occludin and claudin-5 immunoreactivity were decreased while truncation of β-dystroglycan, as well as FITC-dextran and water extravasation was increased. All changes other than β-dystroglycan and edema persisted 7 days later, occurred with increases in GFAP and COX-2, and were blocked by ketoprofen after Meth treatment. In addition, persistent increases in FITC-dextran extravasation were prevented by treatment with an EP1 receptor antagonist after Meth exposure. The results indicate that CUS and Meth synergize to produce long-lasting structural and functional BBB disruptions that are mediated by cyclooxygenase and protracted increases in inflammation. These results suggest that stress and Meth can synergize to produce a long-lasting vulnerability of the brain to subsequent environmental insults resulting from the persistent breach of the BBB.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflict of interest.
Figures
Fig. 1
Rectal temperatures after exposure to stress and Meth. Meth or saline was administered to previously stressed or control rats. Body temperatures were measured before and every hr after a Meth or saline injection, indicated by the arrows on the x-axis. Meth significantly increased body temperature over time (*, p<0.01) and prior exposure to 10 days of CUS significantly increased body temperature compared to No Stress + Saline (#, p<0.001) and No Stress + Meth ($, p<0.05). (_n_=8–10 in each group)
Fig. 2
Alterations in BBB structural proteins 24 hrs after treatment. Meth or saline was administered to previously stressed or control rats. Occludin and claudin-5 protein expression was measured in isolated brain capillaries via Western Blot. a) The combination of stress and Meth significantly decreased occludin immunoreactivity (*, p<0.01, compared to Stress + Saline and No Stress + Meth) (_n_=8–11 in each group) b) Exposure to stress significantly decreased claudin-5 immunoreactivity (*, _p_=0.01, compared to No Stress treated groups). (_n_=8–11 in each group) c) Representative Western Blot images illustrating occludin (57 kDa), claudin-5 (22 kDa) and α-tubulin (50 kDa) immunoreactive bands. d) Stress and Meth increased truncation of β-dystroglycan, compared to No Stress + Saline (*, p<0.05) and Stress + Meth significantly enhanced truncation of β-dystroglycan compared to No Stress + Meth and Stress + Saline (&, p<0.001). (_n_=5–7 in each group) e) Representative Western Blot images illustrating full-form (40 kDa) and truncated β-dystroglycan (22 kDa) immunoreactive bands
Fig. 3
Changes in brain water content, 24 hrs after treatment. Meth or saline was administered to previously stressed or control rats. The combination of stress and Meth significantly increased brain water content, compared to all other groups (*, p<0.01). (_n_=8–12 in each group)
Fig. 4
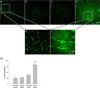
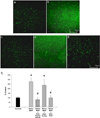
Alterations in BBB permeability to FITC-dextran, 24 hrs after treatment. Meth or saline was administered to previously stressed or control rats. Extravasation of 10,000 dalton FITC-dextran was assessed 24 hrs after treatment. a–f) Representative 20× images of FITC-dextran in the cortex illustrate brightly green stained capillaries against a black background in the a) No Stress + Saline, b) Stress + Saline and c) No Stress + Meth treated groups, and brighter green fluorescence in tissue adjacent to the capillaries in the d) Stress + Meth group (scale bar: 250 µm). e–f) The inset figures illustrate areas of distinct leakage that were apparent in the f) Stress + Meth group only (scale bar: 50 µm). g) Quantification of FITC-dextran extravasation in the cortex, 24 hrs after treatment. The combination of stress and Meth significantly increased FITC-dextran extravasation in the cortex compared to all other groups (*, p<0.05). (_n_=3 in each group)
Fig. 5
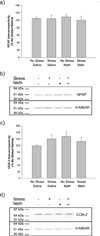
Markers of neuroinflammation, 24 hrs after treatment. Meth or saline was administered to previously stressed or control rats. GFAP and COX-2 protein expression was measured in cortex. a) GFAP immunoreactivity was not altered by stress or Meth. (_n_=6 in each group) b) Representative Western Blot images illustrating GFAP (50 kDa) and α-tubulin (50 kDa) immunoreactive bands. c) COX-2 immunoreactivity was not altered by stress or Meth. (_n_=6 in each group) d) Representative Western Blot images illustrating COX-2 (72 kDa) and α-tubulin (50 kDa) immunoreactive bands
Fig. 6
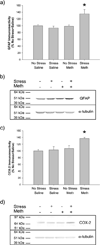
Markers of neuroinflammation, 7 days after treatment. Meth or saline was administered to previously stressed or control rats. GFAP and COX-2 protein expression was measured in cortex. a) GFAP immunoreactivity was significantly increased by the combination of stress and Meth compared to all other groups (*, p<0.05). (_n_=5–8 in each group) b) Representative Western Blot images illustrating GFAP (50 kDa) and α-tubulin (50 kDa) immunoreactive bands. c) COX-2 immunoreactivity was significantly increased by the combination of stress and Meth compared to all other groups (*, p<0.05). (_n_=5–8 in each group) d) Representative Western Blot images illustrating COX-2 (72 kDa) and α-tubulin (50 kDa) immunoreactive bands
Fig. 7
COX-2 protein expression in isolated brain capillaries, 7 days after treatment. Meth or saline was administered to previously stressed or control rats. Ketoprofen was administered to some animals during, after or during and after the Meth or saline treatment. COX-2 protein expression was measured in isolated capillaries. a) COX-2 immunoreactivity was significantly increased by the combination of stress and Meth compared to Controls (No Stress + Saline treated rats regardless of ketoprofen treatment) (*, p<0.001). COX-2 increases were prevented when ketoprofen was administered during and after (#, p<0.001) or after Stress + Meth (#, p<0.001). (_n_=6–12 for all Stress + Meth groups, _n_=19 for Controls) b) Representative Western Blot images illustrating COX-2 (72 kDa) and α-tubulin (50 kDa) immunoreactive bands
Fig. 8
Rectal temperatures after exposure to stress, Meth and ketoprofen. Meth or saline was administered to previously stressed or control rats. Some rats received ketoprofen 1 hr before each Meth or saline injection (Ketoprofen During). Those rats that received ketoprofen post-treatment only, have not yet been exposed to ketoprofen. Body temperatures were measured before and every hr after a Meth or saline injection. Larger arrows on the x-axis indicate the time of Meth or saline injections. The smaller arrows indicate ketoprofen or vehicle injections. The combination of stress and Meth significantly increased body temperature over time (*, p<0.001) and ketoprofen had no effect on Stress + Meth-induced hyperthermia. (_n_=9–16 in each group)
Fig. 9
Long-term alterations in BBB transmembrane structural proteins, 7 days after treatment. Meth or saline was administered to previously stressed or control rats. Some rats received ketoprofen during, after or during and after the Meth or saline treatment. Occludin and claudin-5 protein expression was assessed 7 days after treatment. Stress + Meth significantly decreased a) occludin and b) claudin-5 immunoreactivity and ketoprofen administered during and after or after treatment prevented the occludin decreases (*, p<0.01, compared to Controls; #, p<0.05, compared to Stress + Meth). (_n_=7–16 in each group) c) Representative Western Blot images illustrating occludin (57 kDa), claudin-5 (22 kDa) and α-tubulin (50 kDa) immunoreactive bands
Fig. 10
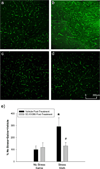
Long-term alterations in BBB permeability to FITC-dextran, 7 days after treatment, are prevented by ketoprofen administration after treatment. Meth or saline was administered to previously stressed or control rats. Some rats received ketoprofen during, after or during and after the Meth or saline treatment. Extravasation of 10,000 dalton FITC-dextran was assessed 7 days after treatment. a–e) Representative 20× images of FITC-dextran in the cortex illustrate brightly green stained capillaries against a black background in the a) Control, c) Stress + Meth + Ketoprofen During & Post treatment, and e) Stress + Meth + Ketoprofen Post treatment, while there was a brighter green fluorescence in tissue adjacent to the capillaries in the b) Stress + Meth and d) Stress + Meth + Ketoprofen During treatment groups. (scale bar: 250 µm) f) Quantification of FITC-dextran extravasation in the cortex, 7 days after treatment. The combination of stress and Meth significantly increased FITC-dextran extravasation in the cortex compared to Controls and this increase was prevented by ketoprofen administered during and after or after treatment (*, p<0.01, compared to Controls; #, p<0.05, compared to Stress + Meth). (_n_=3 in each group)
Fig. 11
Long-term alterations in BBB permeability to FITC-dextran, 7 days after treatment, are prevented by SC-51089 administration after treatment. Meth or saline was administered to previously stressed or control rats. Some rats received SC-51089 twice a day for 6 days after Meth or saline treatment. Extravasation of 10,000 dalton FITC-dextran was assessed 7 days after treatment. a–d) Representative 20× images of FITC-dextran in the cortex illustrate brightly green stained capillaries against a black background in the a) No Stress + Saline + Vehicle, c) No Stress + Saline + SC-51089 and d) Stress + Meth + SC-51089, while there was a brighter green fluorescence in tissue adjacent to the capillaries in the b) Stress + Meth + Vehicle treated group. (scale bar: 250 µm) f) Quantification of FITC-dextran extravasation in the cortex, 7 days after treatment. The combination of stress and Meth significantly increased FITC-dextran extravasation in the cortex compared to No Stress + Saline + Vehicle (*, p<0.01) and this increase was prevented by SC-51089 post-treatment (#, p< 0.05, compared to Stress + Meth). (_n_=3–4 in each group)
Similar articles
- Cyclooxygenase activity contributes to the monoaminergic damage caused by serial exposure to stress and methamphetamine.
Northrop NA, Yamamoto BK. Northrop NA, et al. Neuropharmacology. 2013 Sep;72:96-105. doi: 10.1016/j.neuropharm.2013.04.040. Epub 2013 May 2. Neuropharmacology. 2013. PMID: 23643743 Free PMC article. - Peripheral ammonia and blood brain barrier structure and function after methamphetamine.
Northrop NA, Halpin LE, Yamamoto BK. Northrop NA, et al. Neuropharmacology. 2016 Aug;107:18-26. doi: 10.1016/j.neuropharm.2016.03.018. Epub 2016 Mar 10. Neuropharmacology. 2016. PMID: 26972828 Free PMC article. - Cerebrovascular Injury After Serial Exposure to Chronic Stress and Abstinence from Methamphetamine Self-Administration.
Natarajan R, Mitchell CM, Harless N, Yamamoto BK. Natarajan R, et al. Sci Rep. 2018 Jul 12;8(1):10558. doi: 10.1038/s41598-018-28970-1. Sci Rep. 2018. PMID: 30002494 Free PMC article. - Breakdown of Blood-Brain and Blood-Spinal Cord Barriers During Acute Methamphetamine Intoxication: Role of Brain Temperature.
Kiyatkin EA, Sharma HS. Kiyatkin EA, et al. CNS Neurol Disord Drug Targets. 2016;15(9):1129-1138. doi: 10.2174/1871527315666160920112445. CNS Neurol Disord Drug Targets. 2016. PMID: 27658516 Free PMC article. Review. - Leakage of the blood-brain barrier followed by vasogenic edema as the ultimate cause of death induced by acute methamphetamine overdose.
Kiyatkin EA, Sharma HS. Kiyatkin EA, et al. Int Rev Neurobiol. 2019;146:189-207. doi: 10.1016/bs.irn.2019.06.010. Epub 2019 Jul 9. Int Rev Neurobiol. 2019. PMID: 31349927 Free PMC article. Review.
Cited by
- Acetyl-L-Carnitine Prevents Methamphetamine-Induced Structural Damage on Endothelial Cells via ILK-Related MMP-9 Activity.
Fernandes S, Salta S, Bravo J, Silva AP, Summavielle T. Fernandes S, et al. Mol Neurobiol. 2016 Jan;53(1):408-422. doi: 10.1007/s12035-014-8973-5. Epub 2014 Dec 3. Mol Neurobiol. 2016. PMID: 25465237 - Cyclooxygenase activity contributes to the monoaminergic damage caused by serial exposure to stress and methamphetamine.
Northrop NA, Yamamoto BK. Northrop NA, et al. Neuropharmacology. 2013 Sep;72:96-105. doi: 10.1016/j.neuropharm.2013.04.040. Epub 2013 May 2. Neuropharmacology. 2013. PMID: 23643743 Free PMC article. - Microglia in neuroimmunopharmacology and drug addiction.
Li H, Watkins LR, Wang X. Li H, et al. Mol Psychiatry. 2024 Jun;29(6):1912-1924. doi: 10.1038/s41380-024-02443-6. Epub 2024 Feb 2. Mol Psychiatry. 2024. PMID: 38302560 Review. - Claudin-5: gatekeeper of neurological function.
Greene C, Hanley N, Campbell M. Greene C, et al. Fluids Barriers CNS. 2019 Jan 29;16(1):3. doi: 10.1186/s12987-019-0123-z. Fluids Barriers CNS. 2019. PMID: 30691500 Free PMC article. Review.
References
- Asanuma M, Tsuji T, Miyazaki I, Miyoshi K, Ogawa N. Methamphetamine-induced neurotoxicity in mouse brain is attenuated by ketoprofen, a non-steroidal anti-inflammatory drug. Neurosci Lett. 2003;352:13–16. - PubMed
- Barichello T, Lemos JC, Generoso JS, Cipriano AL, Milioli GL, Marcelino DM, Vuolo F, Petronilho F, Dal-Pizzol F, Vilela MC, Teixeira AL. Oxidative stress, cytokine/chemokine and disruption of blood–brain barrier in neonate rats after meningitis by streptococcus agalactiae. Neurochem Res. 2011 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous