Cas5d protein processes pre-crRNA and assembles into a cascade-like interference complex in subtype I-C/Dvulg CRISPR-Cas system - PubMed (original) (raw)
Cas5d protein processes pre-crRNA and assembles into a cascade-like interference complex in subtype I-C/Dvulg CRISPR-Cas system
Ki Hyun Nam et al. Structure. 2012.
Abstract
Clustered regularly interspaced short palindromic repeats (CRISPRs), together with an operon of CRISPR-associated (Cas) proteins, form an RNA-based prokaryotic immune system against exogenous genetic elements. Cas5 family proteins are found in several type I CRISPR-Cas systems. Here, we report the molecular function of subtype I-C/Dvulg Cas5d from Bacillus halodurans. We show that Cas5d cleaves pre-crRNA into unit length by recognizing both the hairpin structure and the 3' single stranded sequence in the CRISPR repeat region. Cas5d structure reveals a ferredoxin domain-based architecture and a catalytic triad formed by Y46, K116, and H117 residues. We further show that after pre-crRNA processing, Cas5d assembles with crRNA, Csd1, and Csd2 proteins to form a multi-sub-unit interference complex similar to Escherichia coli Cascade (CRISPR-associated complex for antiviral defense) in architecture. Our results suggest that formation of a crRNA-presenting Cascade-like complex is likely a common theme among type I CRISPR subtypes.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures
Figure 1. Cas5d is the pre-crRNA processor in the Subtype I-C/Dvulg CRISPR-Cas system
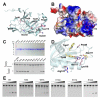
(A) Diagram of the CRISPR/Cas system in B. halodurans C-125, which consists of three CRISPR loci, four core cas genes (cas1, cas2, cas3, and cas4), three Subtype I-C subtype specific cas genes (csd1, csd2 and cas5d), and a Subtype III-B cas genes. The organization and representative sequence of the CRISPR 3 locus is illustrated. (B) SDS-PAGE of the purified subtype-specific B. halodurans Csd1, Csd2 and Cas5d proteins. (C) Pre-crRNA processing assay identified processing activity in Cas5d, but not in Csd1 or Csd2. 5′-HEX-labeled CRISPR repeat RNA (0.2 μM) was used as the substrate with a titration of purified Csd1, Csd2, Cas5d, or equimolar amount of three proteins together (0.04, 0.2 and 1.0 μM). Cas5 cleaved between G21 and U22 in the 32-nt repeat sequence as indicated by the alkaline hydrolysis (OH) sequence ladder. (D) Metal ion-independent endoribonuclease activity of Cas5d. Cleavage reaction was carried out in the presence of 10 mM Mg2+, Mn2+, Ca2+, Ni2+, Zn2+, or EDTA. (E) To verify the presence of a 2′,3′-cyclic phosphate in the 5′-half of the crRNA product, 0.2 μM 5′-HEX were incubated with 1 μM Cas5d at 37 °C for 20 min, the reactions were then further incubated with either T4 polynucleotide kinase (PNK) or Calf Intestine Phosphotase (CIP) for an additional 30 min, separated on 18% sequencing gel, and scanned using typhoon 2900. T4 PNK, but not CIP, is capable of removing the 2′,3′-cyclic phosphate, causing the 5′ product to migrate slower. (F) To verify the presence of a 5′-OH in the 3′-half of the cleavage product, the 3′-Fluoresein labeled pre-crRNA repeats were incubated with Cas5d in similar reaction condition. Further incubation with T4 PNK in the presence of ATP added a phosphate to the 5′-OH and caused the 3′ product to migrate slightly faster. See also Figure S1.
Figure 2. Substrate specificity in Cas5d probed using synthetic pre-crRNA constructs
(A) Secondary structure of the CRISPR repeats in the pre-crRNA. Consensus and variable sequences are in black and red, respectively. (B) Conservation among the B. halodurans CRISPR RNA repeats shown in the form of sequence alignment [top, a tabulation of the first (F) and last (L) repeat in each CRISPR locus] and sequence logo (bottom). (C) Cas5d activity on pre-crRNAs containing sequence substitutions. 0.2 μM chemically synthesized RNAs with either 5′-HEX (red asterisk) or 3′-fluorescein (green asterisk) label were incubated at 25 °C for 20 minutes with increasing concentration of Cas5d (left to right: 0, 1, 2, 3 and 5 μM). Cleavage efficiency was analyzed using a 15% urea-PAGE gel, annotated with the sequence and secondary structure of each RNA. Base substitutions are highlighted in red. Detailed pre-crRNA processing activities are documented in Table S1.
Figure 3. Crystal structure of Csd5d
(A) Cas5d consists of an N-terminal ferredoxin domain (β1- β6; cyan) with an additional β-sheet insertion (β3-β4; yellow). This insertion and a C-terminal twisted β-sheet (β7-β10; pink) form a “wall” fencing the ferredoxin domain (see the side view to the right). An unstructured C-terminal tail interacts with the ferredoxin fold of an adjacent molecule. (B) Surface representation of Cas5d highlighting the putative pre-crRNA binding pocket (in yellow). The “walls” by the β-sheet insertions were colored in gold, and the catalytic triad in red. (C) Top: purity of the Cas5d deletion mutants after purification. Bottom: Deletion mapping showing that the twisted β-sheet is required for the pre-crRNA cleavage activity of Cas5d, whereas the C-terminal tail is not. 0.2 μM of proteins and 0.2 μM of 5′-Hex labeled pre-crRNA were incubated with Cas5d proteins for 20 min at 25°C. See also Figure S2.
Figure 4. Identification of catalytic center in Cas5d via alanine scanning
(A) Cartoon and (B) electrostatic surface representation of Cas5d highlighting the conserved surface residues targeted for alanine substitutions. (C) Top: purity of the Cas5d mutants after purification. Bottom: Cleavage activity of the Cas5d mutants. 0.2 μM wild-type or mutant Cas5d protein was incubated with 0.2 μM 5′-HEX labeled pre-crRNA repeat sequence for 20 min at 25°C. Reaction products were phenol-extracted and a nalyzed on urea-PAGE. Y46A, W47A, K116A, H117A and R123A mutants showed reduced pre-crRNA processing activity to various extent. (D) Putative catalytic triad in Cas5d. Tyr46, Lys116 and His117 residues form a charge relay network mediated by the hydrogen bonding network. The nearby Trp47 forms conserved Π-Π stacking with P49 residue. It is conceivable that Trp47 further stacks near the bottom of the pre-crRNA stemloop, positioning the 3′-tail to the catalytic triad. The conserved Arg123 residue is ~9 Å from the triad, it may move closer towards the catalytic center upon RNA binding. (E) Quantification of the mutational effect using Cas5d titrations. 5′-HEX-labeled CRISPR repeat RNA (0.2 μM) was used as the substrate with a titration of the purified mutant Cas5d proteins (0, 0.1, 0.2, 0.4 and 1.0 μM). See also Figure S3.
Figure 5. Reconstruction of Subtype I-C/Dvulg Cascade complex
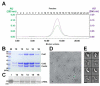
(A) Superose 6 SEC elution profile of the B. halodurans Cascade complex reconstituted from coexpressing Csd1, Csd2, Cas5d, and crRNA in E. coli. The molecular weight is ~400 kDa as compared to the MW standards. Analysis of the fractions in panel (A) was done using (B) Coomassie blue-stained SDS-PAGE gel or (C) SYBR-GOLD-stained denaturing gel. Fraction numbers are consistent with those shown in panel (A). (D) Negative staining electron micrograph of the B. halodurans Cascade. (E) Two major species were identified from the 2D classification (odd rows, corresponding raw images were shown underneath in even rows). The smaller complex (bottom two rows) likely lacked the Csd1 subunit (indicated by the arrows on the top two rows). Scale bars correspond to 10 nm. See also Figure S4.
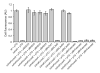
Fig. 6. Cas-dependent silencing of ssTorA-GFP in E. coli
Cellular fluorescence of wild-type (wt) E. coli BW25113, BW25113 Δ_dnaK_, and isogenic cas mutant strains (e.g., BW25113 Δ_dnaK_Δ_casE_) expressing ssTorA-GFP from plasmid pTG. Complementation of silencing activity was assayed using pBhCsd1, pBhCsd2, pBhCas5d or pBhCascade (Cas5d/Csd1/Csd2) and compared to the empty vector (pBAD33) control. Data is the average of three replicate experiments and error is reported as the standard error of the mean.
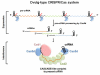
Figure 7. Mechanistic model for crRNA-mediated DNA silencing in Subtype I-C/Dvulg CRISPR-Cas system
The pre-crRNA transcribed from the CRISPR loci is processed by Cas5d into mature crRNAs, each containing a spacer sequence between the 5′ and 3′ handles. Subsequently, Subtype I-C/Dvulg Cascade is formed from Cas5d, Csd1, and Csd2 proteins to present the crRNA in an extended conformation, initiating the process to invade and pair with the complementary ds-DNA, followed by ds-DNA degradation by Cas3. The stoichiometry and the location of each component were envisioned based on data generated here combined with knowledge from the E. coli Cascade (Brouns et al., 2008; Jore et al., 2011; Wiedenheft et al., 2011).
Comment in
- Defense systems up: structure of subtype I-C/Dvulg CRISPR/Cas.
Laronde-Leblanc NA. Laronde-Leblanc NA. Structure. 2012 Sep 5;20(9):1450-2. doi: 10.1016/j.str.2012.08.015. Structure. 2012. PMID: 22958640
Similar articles
- Conservation and variability in the structure and function of the Cas5d endoribonuclease in the CRISPR-mediated microbial immune system.
Koo Y, Ka D, Kim EJ, Suh N, Bae E. Koo Y, et al. J Mol Biol. 2013 Oct 23;425(20):3799-810. doi: 10.1016/j.jmb.2013.02.032. Epub 2013 Mar 7. J Mol Biol. 2013. PMID: 23500492 - Cas5d processes pre-crRNA and is a member of a larger family of CRISPR RNA endonucleases.
Garside EL, Schellenberg MJ, Gesner EM, Bonanno JB, Sauder JM, Burley SK, Almo SC, Mehta G, MacMillan AM. Garside EL, et al. RNA. 2012 Nov;18(11):2020-8. doi: 10.1261/rna.033100.112. Epub 2012 Sep 24. RNA. 2012. PMID: 23006625 Free PMC article. - The CRISPR-associated DNA-cleaving enzyme Cpf1 also processes precursor CRISPR RNA.
Fonfara I, Richter H, Bratovič M, Le Rhun A, Charpentier E. Fonfara I, et al. Nature. 2016 Apr 28;532(7600):517-21. doi: 10.1038/nature17945. Epub 2016 Apr 20. Nature. 2016. PMID: 27096362 - Biogenesis pathways of RNA guides in archaeal and bacterial CRISPR-Cas adaptive immunity.
Charpentier E, Richter H, van der Oost J, White MF. Charpentier E, et al. FEMS Microbiol Rev. 2015 May;39(3):428-41. doi: 10.1093/femsre/fuv023. Epub 2015 May 19. FEMS Microbiol Rev. 2015. PMID: 25994611 Free PMC article. Review. - Cutting it close: CRISPR-associated endoribonuclease structure and function.
Hochstrasser ML, Doudna JA. Hochstrasser ML, et al. Trends Biochem Sci. 2015 Jan;40(1):58-66. doi: 10.1016/j.tibs.2014.10.007. Epub 2014 Nov 18. Trends Biochem Sci. 2015. PMID: 25468820 Review.
Cited by
- The CRISPR-associated gene cas2 of Legionella pneumophila is required for intracellular infection of amoebae.
Gunderson FF, Cianciotto NP. Gunderson FF, et al. mBio. 2013 Mar 12;4(2):e00074-13. doi: 10.1128/mBio.00074-13. mBio. 2013. PMID: 23481601 Free PMC article. - Structural snapshots of R-loop formation by a type I-C CRISPR Cascade.
O'Brien RE, Bravo JPK, Ramos D, Hibshman GN, Wright JT, Taylor DW. O'Brien RE, et al. Mol Cell. 2023 Mar 2;83(5):746-758.e5. doi: 10.1016/j.molcel.2023.01.024. Epub 2023 Feb 16. Mol Cell. 2023. PMID: 36805026 Free PMC article. - Structure and RNA-binding properties of the type III-A CRISPR-associated protein Csm3.
Hrle A, Su AA, Ebert J, Benda C, Randau L, Conti E. Hrle A, et al. RNA Biol. 2013 Nov;10(11):1670-8. doi: 10.4161/rna.26500. Epub 2013 Sep 30. RNA Biol. 2013. PMID: 24157656 Free PMC article. - Develop a Compact RNA Base Editor by Fusing ADAR with Engineered EcCas6e.
Wang X, Zhang R, Yang D, Li G, Fan Z, Du H, Wang Z, Liu Y, Lin J, Wu X, Shi L, Yang H, Zhou Y. Wang X, et al. Adv Sci (Weinh). 2023 Jun;10(17):e2206813. doi: 10.1002/advs.202206813. Epub 2023 Apr 25. Adv Sci (Weinh). 2023. PMID: 37098587 Free PMC article. - Overview of CRISPR-Cas9 Biology.
Ratner HK, Sampson TR, Weiss DS. Ratner HK, et al. Cold Spring Harb Protoc. 2016 Dec 1;2016(12):pdb.top088849. doi: 10.1101/pdb.top088849. Cold Spring Harb Protoc. 2016. PMID: 27934695 Free PMC article.
References
- Barrangou R, Fremaux C, Deveau H, Richards M, Boyaval P, Moineau S, Romero DA, Horvath P. CRISPR provides acquired resistance against viruses in prokaryotes. Science. 2007;315:1709–1712. - PubMed
- Bolotin A, Quinquis B, Sorokin A, Ehrlich SD. Clustered regularly interspaced short palindrome repeats (CRISPRs) have spacers of extrachromosomal origin. Microbiology. 2005;151:2551–2561. - PubMed
- Brunger AT, Adams PD, Clore GM, DeLano WL, Gros P, Grosse-Kunstleve RW, Jiang JS, Kuszewski J, Nilges M, Pannu NS, et al. Crystallography & NMR system: A new software suite for macromolecular structure determination. Acta Crystallogr D Biol Crystallogr. 1998;54:905–921. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- GM-059604/GM/NIGMS NIH HHS/United States
- R01 GM059604/GM/NIGMS NIH HHS/United States
- R01 GM086766/GM/NIGMS NIH HHS/United States
- P41 RR001646/RR/NCRR NIH HHS/United States
- GM-086766/GM/NIGMS NIH HHS/United States
- P41 RR015301/RR/NCRR NIH HHS/United States
- R01 GM102543/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases