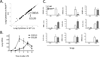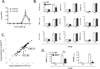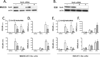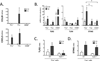Type I alveolar epithelial cells mount innate immune responses during pneumococcal pneumonia - PubMed (original) (raw)
Type I alveolar epithelial cells mount innate immune responses during pneumococcal pneumonia
Kazuko Yamamoto et al. J Immunol. 2012.
Abstract
Pneumonia results from bacteria in the alveoli. The alveolar epithelium consists of type II cells, which secrete surfactant and associated proteins, and type I cells, which constitute 95% of the surface area and meet anatomic and structural needs. Other than constitutively expressed surfactant proteins, it is unknown whether alveolar epithelial cells have distinct roles in innate immunity. Because innate immunity gene induction depends on NF-κB RelA (also known as p65) during pneumonia, we generated a murine model of RelA mutated throughout the alveolar epithelium. In response to LPS, only 2 of 84 cytokine transcripts (CCL20 and CXCL5) were blunted in lungs of mutants, suggesting that a very limited subset of immune mediators is selectively elaborated by the alveolar epithelium. Lung CCL20 induction required epithelial RelA regardless of stimulus, whereas lung CXCL5 expression depended on RelA after instillation of LPS but not pneumococcus. RelA knockdown in vitro suggested that CXCL5 induction required RelA in type II cells but not type I cells. Sorted cell populations from mouse lungs revealed that CXCL5 was induced during pneumonia in type I cells, which did not require RelA. TLR2 and STING were also induced in type I cells, with RelA essential for TLR2 but not STING. To our knowledge, these data are the first direct demonstration that type I cells, which constitute the majority of the alveolar surface, mount innate immune responses during bacterial infection. These are also, to our knowledge, the first evidence for entirely RelA-independent pathways of innate immunity gene induction in any cell during pneumonia.
Figures
Figure 1. A small subset of cytokines is decreased in the absence of alveolar epithelial RelA during LPS-induced pulmonary inflammation
A. Lung inflammatory cytokine mRNA expression after 6h of intratracheal LPS in presence or absence of functional RelA in alveolar epithelial cells (SPC-rtTAwt/tg tetO-Cretg/tg RelAF/F). A total of 84 cytokine-related mRNA levels were determined using PCR array and data for the each group were expressed as Ct. Data shown are results from lung RNA pooled from 6 mice in each group. B. Lung inflammatory cytokine mRNA expression (CXCL5 and CCL20) over a time-course after intratracheal LPS instillation, measured in C57BL/6 mice using qRT-PCR (n=3–5 in each time-point, collected over 2 independent experiments). C. Lung inflammatory cytokine mRNA expression after 0h or 6h of intratracheal LPS were measured by qRT-PCR in presence and absence of functional RelA in alveolar epithelial cells. WT, wild-type non-targeted mice (tetO-Cretg/tg RelAF/F, no Cre transgene); RelAΔ/Δ, transgenic mice with mutant RelA in alveolar epithelial cells (SPC-rtTAwt/tg tetO-Cretg/tg RelAF/F). *, p < 0.05 compared with WT mice. n=3–4 mice/group in Ctrl, and n=6 mice/group in LPS, collected over 3 independent experiments.
Figure 2. During pneumococcal pneumonia, CCL20 but not CXCL5 requires alveolar epithelial RelA
A. Lung inflammatory cytokine mRNA expression (CXCL5 and CCL20) over a time-course after S. pneumoniae serotype 3 (Sp) instillation, measured in C57BL/6 mice using qRT-PCR (n=3 in each time-point, collected over 2 independent experiments). B. Lung inflammatory cytokine mRNA expression after 0h or 15h of intratracheal Sp were measured by qRT-PCR in presence (WT) or absence of functional RelA (RelAΔ/Δ) in alveolar epithelial cells (n=3–4 uninfected mice/group in Ctrl, and n=5–6 infected mice/group in Sp, collected over 3 independent experiments). C. Lung inflammatory cytokine mRNA expression after 15h of intratracheal Sp in presence (WT) or absence of functional RelA (RelAΔ/Δ) in alveolar epithelial cells. A total of 84 cytokine-related mRNA levels were determined using PCR array and data for the each group were expressed as Ct. Data shown are results from lung RNA pooled from 5–6 mice in each group. D. CXCL5 and CCL20 were measured by ELISA in lung homogenates harvested from uninfected mice and mice intratracheally infected with Sp for 15h, in presence (WT) or absence (RelAΔ/Δ) of functional RelA in alveolar epithelial cells. *, p < 0.05 compared with WT mice (n=3 uninfected/group, and n=3–8 infected/group, collected over 2 independent experiments).
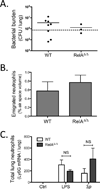
Figure 3. Integrated immunity in mice with alveolar epithelial cell mutations in RelA
A. Viable bacteria in the lung were quantified using CFU assays after 15h pneumococcal infection (n=3–8 mice/group, collected over 2 independent experiments). B. Emigrated neutrophils were measured by quantifying neutrophils in alveolar airspaces using morphometry (n=5–9 mice/group, collected over 3 independent experiments). C. Neutrophil accumulation was evaluated by measuring Ly6G mRNA in lung lysates after 6h of LPS challenge or 15h of pneumococcal infection, expressed as fold WT Ctrl (n=3–4 mice/group in Ctrl, and n=5–6 mice/group in each of LPS and in Sp groups, collected over 3 independent experiments).
Figure 4. CCL20 induction is consistently dependent on RelA, whereas CXCL5 induction is RelA-dependent in AT2-like cells (MLE15) but RelA-independent in AT1-like cells (E10)
A. Immunoblot shows that RelA was knocked down by siRNA in MLE15 cells. Data are representative of n=5 independent experiments. B. Immunoblot shows that RelA was knocked down by siRNA in E10 cells. Actin was used for loading control. Data are representative of n=4 independent experiments. C. CCL20 was measured by qRT-PCR of cell lysates and by ELISA of cell supernatants after 6h of stimulations (TNF-α and LPS) in MLE15 cells in which RelA was knocked down by siRNA. Results reflect data from n=5 independent experiments. D. CXCL5 was measured by qRT-PCR of cell lysates and by ELISA of cell supernatants after 6h of stimulations (TNF-α and LPS) in MLE15 cells in which RelA was knockdown by siRNA. Results reflect data from n=5 independent experiments. E. CCL20 was measured by qRT-PCR of cell lysates and by ELISA of cell supernatants after 6h of stimulations (TNF-α and LPS) in E10 cells in which RelA was knockdown by siRNA. Results reflect data from n=4 independent experiments. F. CXCL5 was measured by qRT-PCR of cell lysates and by ELISA of cell supernatants after 6h of stimulations (TNF-α and LPS) in E10 cells in which RelA was knockdown by siRNA. Results reflect data from n=4 independent experiments. *, p < 0.05 compared with control (non-targeted siRNA).
Figure 5. During pneumococcal pneumonia, CXCL5 is induced in AT1 cells
A. AT1 cells were collected by sorting for surface expression of T1α within lung single cell suspensions from C57BL/6 mice using flow cytometry. Results are representative of n=5 mice collected over 3 independent experiments. B. qRT-PCR of cell-type marker expression amongst T1α+ cells and T1α− cells reveals that they former are positive for AT1 markers (podoplanin and caveolin-1) while markers for AT2 (SP-C, Nkx2-1) or other cells (CC10, PECAM-1, p75) were higher in the latter. Data are from n=5 mice/group, collected over 3 independent experiments. *, p < 0.05 compared with T1α+cells. C. Immunofluorescence images of sorted CD45−T1α+ cells and CD45−T1α− cells demonstrates caveolin-1 staining (characteristic of type I cells) exclusively in the former, and pro-SPC staining (characteristic of type II cells) exclusively in the latter. pro-SP-C, pro-surfactant protein-C. Scale bars: 100µm. Results are representative of n=4 mice collected over 2 independent experiments. D. CCL20 was primarily induced in T1α− cells during LPS-induced pulmonary inflammation in C57BL/6 mice, measured using qRT-PCR (n=3 mice/group in Ctrl, and n=7 mice/group in LPS, collected over 2 independent experiments). E. CXCL5 was primarily induced in T1α− cells during LPS-induced pulmonary inflammation in C57BL/6 mice, measured using qRT-PCR (n=3 mice/group in Ctrl, and n=7 mice/group in LPS, collected over 2 independent experiments). F. CCL20 was primarily induced in T1α− cells during pneumococcal pneumonia in C57BL/6 mice, measured using qRT-PCR (n=4 mice/group in Ctrl, and n=9 mice/group in Sp, collected over 4 independent experiments). G. CXCL5 was primarily induced in T1α+ cells during pneumococcal pneumonia in C57BL/6 mice, measured using qRT-PCR, evidence that AT1 cells respond with innate immunity gene induction during pneumococcal pneumonia (n=4 uninfected mice/group, and n=9 infected mice/group, collected over 4 independent experiments). *, p < 0.05 compared with uninfected control mice. †, p < 0.05 compared with T1α+ cells.
Figure 6. AT1 cell induction of CXCL5 during pneumococcal pneumonia is independent of RelA
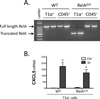
A. RelA is effectively targeted in AT1 cells by transgenesis. RT-PCR of wild-type and mutant RelA allele products in T1α+ cells and CD45+ cells isolated from lung single cell suspension of WT (tetO-Cretg/tg RelAF/F, no Cre transgene) or epithelial mutant RelAΔ/Δ (SPC-rtTAwt/tg tetO-Cretg/tg RelAF/F) mice reveals that the T1α+ cells express the mutant allele, in targeted mice selectively. Results are representative of n=4 mice collected over 2 independent experiments. B. The induction of CXCL5 in T1α+ cells does not require RelA. CXCL5 was measured by qRT-PCR in sorted T1α+ cells from uninfected mice or during Sp pneumonia, using WT or epithelial mutant RelAΔ/Δ mice (n=3 mice/group in Ctrl, and n=6 mice/group in Sp, collected over 3 independent experiments). *, p < 0.05 compared with uninfected control mice.
Figure 7. AT1 cell induction of TLR2 is dependent on RelA, whereas STING induction is RelA-independent
A. Sorting strategy revised to differentiate gene expression from CD45+ leukocytes supports the concept that CCL20 and CXCL5 are exclusively epithelial in origin. The induction of CCL20 and CXCL5 was measured by qRT-PCR in T1α+ cells, T1α−cells, and CD45+ cells collected from uninfected lungs and during Sp pneumonia (n=3 mice/group in Ctrl, and n=6 mice/group in Sp, collected over 3 independent experiments). B. The expression of PRRs (TLR2, TLR4, and STING) was measured using qRT-PCR in T1α+ cells, T1α− cells, and CD45+ cells collected from uninfected lungs and during Sp pneumonia (n=3 mice/group in Ctrl, and n=6 mice/group in Sp, collected over 3 independent experiments). *, p < 0.05 compared with uninfected control mice, †, p < 0.05 compared with T1α+ cells. C. The induction of TLR2 in T1α+ cells, measured by qRT-PCR, was abrogated by RelA mutation during Sp pneumonia, using lungs from WT (tetO-Cretg/tg RelAF/F, no Cre transgene) or epithelial mutant RelAΔ/Δ (SPC-rtTAwt/tg tetO-Cretg/tg RelAF/F) mice, with n=3 mice/group in Ctrl and n=6 mice/group in Sp, collected over 3 independent experiments D. STING was induced in T1α+ cells during Sp pneumonia even if epithelial cells were deficient in RelA, using qRT-PCR to measure STING in lungs from WT or epithelial mutant RelAΔ/Δ mice (n=3 mice/group in Ctrl, and n=6 mice/group in Sp, collected over 3 independent experiments). *, p < 0.05 compared with uninfected control mice. †, p < 0.05 compared with WT mice.
Similar articles
- Earliest innate immune responses require macrophage RelA during pneumococcal pneumonia.
Pittet LA, Quinton LJ, Yamamoto K, Robson BE, Ferrari JD, Algül H, Schmid RM, Mizgerd JP. Pittet LA, et al. Am J Respir Cell Mol Biol. 2011 Sep;45(3):573-81. doi: 10.1165/rcmb.2010-0210OC. Epub 2011 Jan 7. Am J Respir Cell Mol Biol. 2011. PMID: 21216972 Free PMC article. - Roles of lung epithelium in neutrophil recruitment during pneumococcal pneumonia.
Yamamoto K, Ahyi AN, Pepper-Cunningham ZA, Ferrari JD, Wilson AA, Jones MR, Quinton LJ, Mizgerd JP. Yamamoto K, et al. Am J Respir Cell Mol Biol. 2014 Feb;50(2):253-62. doi: 10.1165/rcmb.2013-0114OC. Am J Respir Cell Mol Biol. 2014. PMID: 24010952 Free PMC article. - Functions and regulation of NF-kappaB RelA during pneumococcal pneumonia.
Quinton LJ, Jones MR, Simms BT, Kogan MS, Robson BE, Skerrett SJ, Mizgerd JP. Quinton LJ, et al. J Immunol. 2007 Feb 1;178(3):1896-903. doi: 10.4049/jimmunol.178.3.1896. J Immunol. 2007. PMID: 17237440 Free PMC article. - Of mice and men: innate immunity in pneumococcal pneumonia.
Calbo E, Garau J. Calbo E, et al. Int J Antimicrob Agents. 2010 Feb;35(2):107-13. doi: 10.1016/j.ijantimicag.2009.10.002. Epub 2009 Dec 14. Int J Antimicrob Agents. 2010. PMID: 20005681 Review. - The innate immune response to pneumococcal lung infection: the untold story.
Kadioglu A, Andrew PW. Kadioglu A, et al. Trends Immunol. 2004 Mar;25(3):143-9. doi: 10.1016/j.it.2003.12.006. Trends Immunol. 2004. PMID: 15036042 Review. No abstract available.
Cited by
- Induction of STAT3-Dependent CXCL5 Expression and Neutrophil Recruitment by Oncostatin-M during Pneumonia.
Traber KE, Hilliard KL, Allen E, Wasserman GA, Yamamoto K, Jones MR, Mizgerd JP, Quinton LJ. Traber KE, et al. Am J Respir Cell Mol Biol. 2015 Oct;53(4):479-88. doi: 10.1165/rcmb.2014-0342OC. Am J Respir Cell Mol Biol. 2015. PMID: 25692402 Free PMC article. - Epithelial membrane protein 2 governs transepithelial migration of neutrophils into the airspace.
Lin WC, Gowdy KM, Madenspacher JH, Zemans RL, Yamamoto K, Lyons-Cohen M, Nakano H, Janardhan K, Williams CJ, Cook DN, Mizgerd JP, Fessler MB. Lin WC, et al. J Clin Invest. 2020 Jan 2;130(1):157-170. doi: 10.1172/JCI127144. J Clin Invest. 2020. PMID: 31550239 Free PMC article. - Aquaporin 5 regulates cigarette smoke induced emphysema by modulating barrier and immune properties of the epithelium.
Aggarwal NR, Chau E, Garibaldi BT, Mock JR, Sussan T, Rao K, Rao K, Menon AG, D'Alessio FR, Damarla M, Biswal S, King LS, Sidhaye VK. Aggarwal NR, et al. Tissue Barriers. 2013 Oct 1;1(4):e25248. doi: 10.4161/tisb.25248. Epub 2013 Jun 3. Tissue Barriers. 2013. PMID: 24665410 Free PMC article. - Expression of Piwi protein MIWI2 defines a distinct population of multiciliated cells.
Wasserman GA, Szymaniak AD, Hinds AC, Yamamoto K, Kamata H, Smith NM, Hilliard KL, Carrieri C, Labadorf AT, Quinton LJ, Ai X, Varelas X, Chen F, Mizgerd JP, Fine A, O'Carroll D, Jones MR. Wasserman GA, et al. J Clin Invest. 2017 Oct 2;127(10):3866-3876. doi: 10.1172/JCI94639. Epub 2017 Sep 18. J Clin Invest. 2017. PMID: 28920925 Free PMC article. - Aging-Associated Molecular Changes in Human Alveolar Type I Cells.
Liu X, Zhang X, Liang J, Noble PW, Jiang D. Liu X, et al. J Respir Biol Transl Med. 2024 Sep;1(3):10012. doi: 10.35534/jrbtm.2024.10012. Epub 2024 Jul 22. J Respir Biol Transl Med. 2024. PMID: 39220636 Free PMC article.
References
- Mason RJ. Biology of alveolar type II cells. Respirology. 2006;11(Suppl):S12–S15. - PubMed
- Crapo JD, Barry BE, Gehr P, Bachofen M, Weibel ER. Cell number and cell characteristics of the normal human lung. Am Rev Respir Dis. 1982;126(2):332–337. - PubMed
- McElroy MC, Kasper M. The use of alveolar epithelial type I cell-selective markers to investigate lung injury and repair. Eur Respir J. 2004;24(4):664–673. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL104053/HL/NHLBI NIH HHS/United States
- K99 HL092956/HL/NHLBI NIH HHS/United States
- HL068153/HL/NHLBI NIH HHS/United States
- R01 HL083034/HL/NHLBI NIH HHS/United States
- HL092956/HL/NHLBI NIH HHS/United States
- HL079392/HL/NHLBI NIH HHS/United States
- HL104053/HL/NHLBI NIH HHS/United States
- R01 HL111449/HL/NHLBI NIH HHS/United States
- R01 HL079392/HL/NHLBI NIH HHS/United States
- R00 HL092956/HL/NHLBI NIH HHS/United States
- R01 HL068153/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials