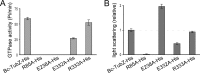Filament formation of the FtsZ/tubulin-like protein TubZ from the Bacillus cereus pXO1 plasmid - PubMed (original) (raw)
Filament formation of the FtsZ/tubulin-like protein TubZ from the Bacillus cereus pXO1 plasmid
Shota Hoshino et al. J Biol Chem. 2012.
Abstract
Stable maintenance of low-copy-number plasmids requires partition (par) systems that consist of a nucleotide hydrolase, a DNA-binding protein, and a cis-acting DNA-binding site. The FtsZ/tubulin-like GTPase TubZ was identified as a partitioning factor of the virulence plasmids pBtoxis and pXO1 in Bacillus thuringiensis and Bacillus anthracis, respectively. TubZ exhibits high GTPase activity and assembles into polymers both in vivo and in vitro, and its "treadmilling" movement is required for plasmid stability in the cell. To investigate the molecular mechanism of pXO1 plasmid segregation by TubZ filaments, we determined the crystal structures of Bacillus cereus TubZ in apo-, GDP-, and guanosine 5'-3-O-(thio)triphosphate (GTPγS)-bound forms at resolutions of 2.1, 1.9, and 3.3 Å, respectively. Interestingly, the slowly hydrolyzable GTP analog GTPγS was hydrolyzed to GDP in the crystal. In the post-GTP hydrolysis state, GDP-bound B. cereus TubZ forms a dimer by the head-to-tail association of individual subunits in the asymmetric unit, which is similar to the protofilament formation of FtsZ and B. thuringiensis TubZ. However, the M loop interacts with the nucleotide-binding site of the adjacent subunit and stabilizes the filament structure in a different manner, which indicates that the molecular assembly of the TubZ-related par systems is not stringently conserved. Furthermore, we show that the C-terminal tail of TubZ is required for association with the DNA-binding protein TubR. Using a combination of crystallography, site-directed mutagenesis, and biochemical analysis, our results provide the structural basis of the TubZ polymer that may drive DNA segregation.
Figures
FIGURE 1.
Crystal structure of Bc-TubZ. A and B, schematic representations of apo-Bc-TubZΔ and GDP-Bc-TubZΔ, respectively. α-Helices are colored green, and β-strands are colored orange. Secondary structural elements are labeled in A. The active site loops (T1–T7) and loops on the other side of the molecule (M, T0, and T7) are labeled in B. GDP is shown as a space-filling model. C, close-up view of the active site in the GDP-bound form. The nucleotide recognition loops are colored cyan. Interacting residues are shown as sticks. D, conformational change of H11. The apo-bound form is colored yellow, and the nucleotide-bound form is colored green. The hinge residue Leu-362 is labeled (red arrowhead). E, stereo view of the interaction between H5 and H11. Residues involved in the interaction are labeled and shown as sticks (Glu-161 in magenta, Lys-377 in blue, and hydrophobic residues in yellow). This figure was made using PyMOL (38).
FIGURE 2.
Structural comparison of Bc-TubZ with Bt-TubZ and Cb-TubZ (A) and with M. jannaschii FtsZ (B). A, superposition of GTPγS-Bc-TubZΔ (green), GTPγS-Bt-TubZ (cyan; Protein Data Bank code 3M89), and apo-Cb-TubZ (purple; code 3V3T). GTPγS is shown as sticks. B, superposition of GDP-Bc-TubZΔ (green) and FtsZ (pink; code 1W59). The H0 and H1 helices are shown as cylinders. Structures are superimposed using the N-terminal GTPase domain. The views are in the same orientation as in Fig. 1_A. Mj_, M. jannaschii.
FIGURE 3.
Protofilament interaction in Bc-TubZ. A, semicontinuous GDP-Bc-TubZΔ protofilament structure in the crystal lattice. The N-terminal GTPase domain is shown in green, the H7 helix in yellow, and C-terminal domain in magenta. The molecular spacing of 45 Å is shown in the middle, whereas the subunit interfaces with less interaction at the are shown at the top and bottom (52-Å spacing). Note that the 45-Å spacing is similar to that observed in the Bt-TubZ crystal structure. The regions containing the subunit-subunit interactions with the 45-Å spacing are boxed. B–D, stereo views of the GDP-binding site, the vicinity of Phe-313, and the C-terminal tail region, respectively. Residues described under “Results” are shown as sticks. In D, Arg-333 is exposed to solvent and makes few contacts with residues in H11. E and F, structural comparison of the GTPγS-Bt-TubZ and FtsZ dimers, respectively. The dimers of GTPγS-Bt-TubZ (chains D and E) (E) and FtsZ (F) observed in the crystal structures are shown. The orientation of the molecules and the color scheme are the same as for GDP-Bc-TubZΔ in A. The lower subunits are superimposed. The GTPγS-Bt-TubZ dimer is twisted, whereas GDP-Bc-TubZΔ and FtsZ are not.
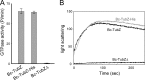
FIGURE 4.
Bc-TubZ GTPase activity and self-assembly. A, GTPase activity of Bc-TubZ, a Bc-TubZ fusion protein with a C-terminal histidine tag (Bc-TubZ-His), and Bc-TubZΔ. Measurements were carried out at protein concentrations of 2 μ
m
. B, the kinetics of Bc-TubZ polymerization were monitored by measuring the increase in light scattering. A protein concentration of 2 μ
m
was used.
FIGURE 5.
GTP hydrolysis and self-assembly of Bc-TubZ mutants. A, the GTPase activities of 2 μ
m
Bc-TubZ-His and its mutants were measured. The R85A and E238A mutations abolished the GTPase activity, whereas the activity of Bc-TubZ-His(E332A) was 40% of that of the wild type. The R333A mutation had no effect on GTPase activity. B, light scattering analyses of Bc-TubZ-His mutants. The relative activity of each reaction was determined by comparing the turbidity after a 60-s incubation. The polymerization activity was consistent with the GTPase activity, except for Bc-TubZ-His(E238A).
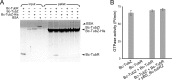
FIGURE 6.
Effect of Bc-TubR and DNA in Bc-TubZ polymerization. A, Bc-TubR binding to Bc-TubZ polymers. Sedimentations of Bc-TubZ and Bc-TubZ-His were performed in the presence or absence of Bc-TubR. BSA binding was also examined as a negative control. B, GTPase activity of 2 μ
m
Bc-TubZ in the presence or absence of 2 μ
m
Bc-TubR and 3 n
m
pBS_Bc-tubRZ.
Similar articles
- Cooperative DNA Binding of the Plasmid Partitioning Protein TubR from the Bacillus cereus pXO1 Plasmid.
Hayashi I, Oda T, Sato M, Fuchigami S. Hayashi I, et al. J Mol Biol. 2018 Dec 7;430(24):5015-5028. doi: 10.1016/j.jmb.2018.11.001. Epub 2018 Nov 8. J Mol Biol. 2018. PMID: 30414406 - Crystallization and preliminary X-ray data analysis of the pXO1 plasmid-partitioning factor TubZ from Bacillus cereus.
Hoshino S, Maki T, Hayashi I. Hoshino S, et al. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2012 Dec 1;68(Pt 12):1550-3. doi: 10.1107/S1744309112045551. Epub 2012 Nov 19. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2012. PMID: 23192045 Free PMC article. - Plasmid protein TubR uses a distinct mode of HTH-DNA binding and recruits the prokaryotic tubulin homolog TubZ to effect DNA partition.
Ni L, Xu W, Kumaraswami M, Schumacher MA. Ni L, et al. Proc Natl Acad Sci U S A. 2010 Jun 29;107(26):11763-8. doi: 10.1073/pnas.1003817107. Epub 2010 Jun 4. Proc Natl Acad Sci U S A. 2010. PMID: 20534443 Free PMC article. - Tubulin-Like Proteins in Prokaryotic DNA Positioning.
Fink G, Aylett CHS. Fink G, et al. Subcell Biochem. 2017;84:323-356. doi: 10.1007/978-3-319-53047-5_11. Subcell Biochem. 2017. PMID: 28500531 Review. - What sets Bacillus anthracis apart from other Bacillus species?
Kolstø AB, Tourasse NJ, Økstad OA. Kolstø AB, et al. Annu Rev Microbiol. 2009;63:451-76. doi: 10.1146/annurev.micro.091208.073255. Annu Rev Microbiol. 2009. PMID: 19514852 Review.
Cited by
- Diversity, origin, and evolution of the ESCRT systems.
Makarova KS, Tobiasson V, Wolf YI, Lu Z, Liu Y, Zhang S, Krupovic M, Li M, Koonin EV. Makarova KS, et al. mBio. 2024 Mar 13;15(3):e0033524. doi: 10.1128/mbio.00335-24. Epub 2024 Feb 21. mBio. 2024. PMID: 38380930 Free PMC article. - Structural visualization of the tubulin folding pathway directed by human chaperonin TRiC/CCT.
Gestaut D, Zhao Y, Park J, Ma B, Leitner A, Collier M, Pintilie G, Roh SH, Chiu W, Frydman J. Gestaut D, et al. Cell. 2022 Dec 8;185(25):4770-4787.e20. doi: 10.1016/j.cell.2022.11.014. Cell. 2022. PMID: 36493755 Free PMC article. - TubZ filament assembly dynamics requires the flexible C-terminal tail.
Fuentes-Pérez ME, Núñez-Ramírez R, Martín-González A, Juan-Rodríguez D, Llorca O, Moreno-Herrero F, Oliva MA. Fuentes-Pérez ME, et al. Sci Rep. 2017 Feb 23;7:43342. doi: 10.1038/srep43342. Sci Rep. 2017. PMID: 28230082 Free PMC article. - Reconstitution of a prokaryotic minus end-tracking system using TubRC centromeric complexes and tubulin-like protein TubZ filaments.
Fink G, Löwe J. Fink G, et al. Proc Natl Acad Sci U S A. 2015 Apr 14;112(15):E1845-50. doi: 10.1073/pnas.1423746112. Epub 2015 Mar 30. Proc Natl Acad Sci U S A. 2015. PMID: 25825718 Free PMC article. - The IntXO-PSL Recombination System Is a Key Component of the Second Maintenance System for Bacillus anthracis Plasmid pXO1.
Pomerantsev AP, Rappole C, Chang Z, Chahoud M, Leppla SH. Pomerantsev AP, et al. J Bacteriol. 2016 Jun 27;198(14):1939-1951. doi: 10.1128/JB.01004-15. Print 2016 Jul 15. J Bacteriol. 2016. PMID: 27137503 Free PMC article.
References
- Carballido-López R., Errington J. (2003) A dynamic bacterial cytoskeleton. Trends Cell Biol. 13, 577–583 - PubMed
- Graumann P. L. (2007) Cytoskeletal elements in bacteria. Annu. Rev. Microbiol. 61, 589–618 - PubMed
- Cabeen M. T., Jacobs-Wagner C. (2010) The bacterial cytoskeleton. Annu. Rev. Genet. 44, 365–392 - PubMed
- van den Ent F., Amos L., Löwe J. (2001) Bacterial ancestry of actin and tubulin. Curr. Opin. Microbiol. 4, 634–638 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources