c-kit+ precursors support postinfarction myogenesis in the neonatal, but not adult, heart - PubMed (original) (raw)
c-kit+ precursors support postinfarction myogenesis in the neonatal, but not adult, heart
Sophy A Jesty et al. Proc Natl Acad Sci U S A. 2012.
Abstract
We examined the myogenic response to infarction in neonatal and adult mice to determine the role of c-kit(+) cardiovascular precursor cells (CPC) that are known to be present in early heart development. Infarction of postnatal day 1-3 c-kit(BAC)-EGFP mouse hearts induced the localized expansion of (c-kit)EGFP(+) cells within the infarct, expression of the c-kit and Nkx2.5 mRNA, myogenesis, and partial regeneration of the infarction, with (c-kit)EGFP(+) cells adopting myogenic and vascular fates. Conversely, infarction of adult mice resulted in a modest induction of (c-kit)EGFP(+) cells within the infarct, which did not express Nkx2.5 or undergo myogenic differentiation, but adopted a vascular fate within the infarction, indicating a lack of authentic CPC. Explantation of infarcted neonatal and adult heart tissue to scid mice, and adoptive transfer of labeled bone marrow, confirmed the cardiac source of myogenic (neonate) and angiogenic (neonate and adult) cells. FACS-purified (c-kit)EGFP(+)/(αMHC)mCherry(-) (noncardiac) cells from microdissected infarcts within 6 h of infarction underwent cardiac differentiation, forming spontaneously beating myocytes in vitro; cre/LoxP fate mapping identified a noncardiac population of (c-kit)EGFP(+) myocytes within infarctions, indicating that the induction of undifferentiated precursors contributes to localized myogenesis. Thus, adult postinfarct myogenic failure is likely not due to a context-dependent restriction of precursor differentiation, and c-kit induction following injury of the adult heart does not define precursor status.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
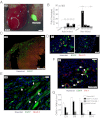
Cardiac myogenesis following infarction of the neonatal heart. (A) Induction of (c-kit)EGFP+ cells in neonatal and adult heart at 3 d after cryoinfarction (D3). Merged fluorescent/bright-field images show localized fluorescence within the neonatal infarct. Adult fluorescent cells not visible at this camera gain. (B) Quantitative PCR (qPCR) of c-kit mRNA in microdissected infarcts. Note higher message in neonatal vs. adult sham, and higher induction in neonatal infarct. Statistical comparisons to group sham. (C) Expression of (c-kit)EGFP in infarcted neonatal heart. (D) Nuclear Nkx2.5 expression in undifferentiated (c-kit)EGFP+ cells in neonatal, but not adult, heart. (E) Striated (c-kit)EGFP+ myocytes within neonatal infarct (arrows) by D3; some cells are also Nkx2.5+. (F) Flk-1+ cells in infarct include (c-kit)EGFP+ cells (arrows). (G) Time course and phenotype of (c-kit)EGFP+ cells following infarction of the neonatal heart. Note predominant cardiac phenotype. Error bars show SEM throughout. (Scale bars: 2,000 μm in A; 100 μm in C; 20 μm in D and E; and 50 μm in F.)
Fig. 2.
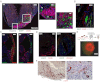
Regeneration of infarcted myocardium through expansion of cardiovascular precursor pool. (A) α-actinin staining at D3, D5, and D21 shows initial fibrosis and clusters of (c-kit)EGFP+ cells, followed by progressive muscle regeneration. (B) Myocyte clusters with many α-actinin+/(c-kit)EGFP+ cells (arrows) at D5 (Left) and fewer (c-kit)EGFP+ myocytes at D21 (Right). (C) Regression of fibrosis and myocyte clusters interspersed within fibrotic regions (trichrome). (D) Marked regeneration at D94, with further regression of fibrotic areas. (E) BrdU incorporation in infarcted neonatal heart. (Inset) BrdU incorporation in a (c-kit)EGFP+ striated myocyte. (F) Quantitative morphometry from BrdU incorporation in all myocytes. (G) Conversion of green to red fluorescence in vitro demonstrating myogenic capacity of (c-kit)EGFP+ cells. Cells shown are at day 14 from an initial plating devoid of red cells. Same field shown as overlay of light and green fluorescence (Upper), and light and red fluoresence (Lower) images. Separation scheme and purity are shown in
Fig. S2
. (H and I) Fate mapping of myocytes (c-kitBAC-EGFP/αMHC-CreER/R26-floxed STOP-βGal) at D5 shows limited recombination in infarct region at low (H) and high (I) magnification (red is autofluorescence). (Scale bars: 400 μm in A Left and Center; 250 μm in A Right; 20 μm in B; 200 μm in C Left; 30 μm in C Right; 1,000 μm in D Left; 25 μm in D Right; 20 μm in E; 10 μm in E Inset; 25 μm in G, 200 μm in H; and 100 μm in I.)
Fig. 3.
Heart–derived (c-kit)EGFP+ cells adopt myogenic and EC fates following neonatal infarction. (A) CD45+ cells surround (c-kit)EGFP+ cells within infarct and do not participate in angiogenesis/myogenesis. (Left) New vessel within infarct (a) formed by (c-kit)EGFP+ endothelial cells; vessel outside of infarct (b) has no incorporation. (Right) Myocyte clusters formed by (c-kit)EGFP+ cells, surrounded by EGFP−/CD45+ mononuclear cells. (B) At D21, some CD45+ cells persist, but are α-actinin negative. (C and D) CD45 and PECAM stained sections from D3 and D21 hearts show distinct, nonvascular location of CD45+ cells within infarcts. (E) Infarction of reconstituted (PN2 c-kitBAC-EGFP/(pCAGGS)dsRed marrow) B6 adults results in homing of only dsRed cells to the infarct (green/red image). (F) (c-kit)EGFP induction and myogenic/endothelial differentiation in neonatal c-kitBAC-EGFP infarcted tissue in SCID mouse. (Left) Low magnification of infarcted area. (Right) Higher magnification shows clusters of striated (c-kit)EGFP+ myocytes (arrows) as well as endothelial cells. (Scale bars: 200 μm in A Left; 50 μm in A Center and Right; 20 μm in B; 200 μm in C; 200 μm in D Left; 50 μm in D Right; 1,000 μm in E; 100 μm in F Left; and 25 μm in F Right.)
Fig. 4.
Heart–derived (c-kit)EGFP+ cells adopt vascular fates following adult infarction. (A) Infarction of reconstituted ((pCAGGS)dsRed marrow) c-kitBAC-EGFP mice indicates (c-kit)EGFP+ cells adopt endothelial (PECAM-1) and smooth muscle (α-smActin) fates; bone marrow-derived cells are mainly CD45+. (B) Infarction of reconstituted (c-kitBAC-EGFP marrow) αMHC-mCherry mice reveals minimal (c-kit)EGFP+ cells homing to the heart (arrow indicates rare EGFP+ cell). (C) CD45+ cells from c-kitBAC-EGFP marrow home to the infarct, but do not form myocytes; mCherry+ myocytes at border zone. (D) Adult c-kitBAC-EGFP infarcted tissue in SCID mouse undergoes (c-kit)EGFP induction, but adopt strictly vascular fates (Right). (E) Induction of sca-1 mRNA in microdissected infarcts. (Scale bars: 500 μm in A Left; 100 μm in A Center and Right; 100 μm in B; 25 μm in C; 200 μm in D Left; and 50 μm in D Right.)
Comment in
- Comment on "Do neonatal mouse hearts regenerate following heart apex resection"?
Kotlikoff MI, Hesse M, Fleischmann BK. Kotlikoff MI, et al. Stem Cell Reports. 2014 Jul 8;3(1):2. doi: 10.1016/j.stemcr.2014.06.010. eCollection 2014 Jul 8. Stem Cell Reports. 2014. PMID: 25068115 Free PMC article. No abstract available.
References
- Sussman MA, Anversa P. Myocardial aging and senescence: Where have the stem cells gone? Annu Rev Physiol. 2004;66:29–48. - PubMed
- Anversa P, Kajstura J, Leri A, Bolli R. Life and death of cardiac stem cells: a paradigm shift in cardiac biology. Circulation. 2006;113:1451–1463. - PubMed
- Rubart M, Field LJ. Cardiac regeneration: Repopulating the heart. Annu Rev Physiol. 2006;68:29–49. - PubMed
- Murry CE, Reinecke H, Pabon LM. Regeneration gaps: Observations on stem cells and cardiac repair. J Am Coll Cardiol. 2006;47:1777–1785. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases