NEMO inhibits programmed necrosis in an NFκB-independent manner by restraining RIP1 - PubMed (original) (raw)
NEMO inhibits programmed necrosis in an NFκB-independent manner by restraining RIP1
Marie Anne O'Donnell et al. PLoS One. 2012.
Abstract
TNF can trigger two opposing responses: cell survival and cell death. TNFR1 activates caspases that orchestrate apoptosis but some cell types switch to a necrotic death when treated with caspase inhibitors. Several genes that are required to orchestrate cell death by programmed necrosis have been identified, such as the kinase RIP1, but very little is known about the inhibitory signals that keep this necrotic cell death pathway in check. We demonstrate that T cells lacking the regulatory subunit of IKK, NFκB essential modifier (NEMO), are hypersensitive to programmed necrosis when stimulated with TNF in the presence of caspase inhibitors. Surprisingly, this pro-survival activity of NEMO is independent of NFκB-mediated gene transcription. Instead, NEMO inhibits necrosis by binding to ubiquitinated RIP1 to restrain RIP1 from engaging the necrotic death pathway. In the absence of NEMO, or if ubiquitination of RIP1 is blocked, necrosis ensues when caspases are blocked. These results indicate that recruitment of NEMO to ubiquitinated RIP1 is a key step in the TNFR1 signaling pathway that determines whether RIP1 triggers a necrotic death response.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
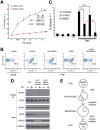
Figure 1. NEMO does not require NFκB to inhibit caspase-independent cell death.
(A) NEMO-deficient (8321) cells and NFκB-deficient (3T8 IκBαSR) cells were co-incubated with 10 µM zVAD-fmk pan-caspase inhibitor and different doses of TNF for 24 h. Cell death was quantified by Annexin V staining and flow cytometry. The mean and SEM of six independent experiments is shown, * denotes p<0.05. (B) Immunoblot indicates the amount of NEMO protein expressed by the parental 3T8 cell line and the two reconstituted clones. (C) Reconstitution of NEMO-deficient cells with NEMO prevents caspase-independent cell death as shown by a TNF dose response of cell death (performed as described in (A) in two clones of NEMO-expressing cells compared to NEMO-deficient cells. (D) Immunoblot of SDS-soluble lysates from NEMO-deficient cells co-incubated with 10 µM zVAD and 10 ng/ml TNF for 24 h indicates that Caspase 3 and PARP cleavage does not occur in NEMO-deficient cells dying in the presence of pan-caspase inhibitor. Genomic DNA was electrophoresed and visualized with ethidium bromide. All cell lines shown in Figure 1 are unable to activate NFκB either due to NEMO deficiency or expression of the IκBαSR.
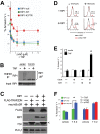
Figure 2. NEMO-deficient cells undergo RIP1 kinase-dependent programmed necrosis.
(A) 8321 NEMO-null cells transduced with retrovirus encoding non-targeting (shNS) or RIP1-targeting short hairpins (shRIP1) were co-incubated with 10 µM zVAD caspase inhibitor and different doses of TNF for 24 h. Cell death was quantified by Annexin V staining and flow cytometry. The mean and SEM of three independent experiments is shown, * denotes p<0.05. Immunoblot indicates efficient knockdown of RIP1 protein in shRIP1 cells. Data shown is representative of similar results obtained with two different RIP1-targeting hairpins. (B) 8321 NEMO-null cells were pre-treated for one hour with 10 µM zVAD and/or 30 µM Necrostatin-1 as indicated, and then stimulated with 10 ng/ml TNF for 24 hours. Cell death was quantified as described in (A). The number inside the polygon gate indicates the percentage of cells that stain with Annexin V. (C) The bar chart displays the mean percentage and SEM from three independent experiments of Annexin V staining cells after 24 h of culture in vehicle, zVAD, Necrostatin-1 or a combination of zVAD and Necrostatin-1 either in the presence or absence of 10 ng/ml TNF for 24 h, * denotes p<0.05. (D) The parental NEMO-sufficient cell line 3T8 and NEMO-deficient 8321 cells transduced with the IκBαSR and reconstituted with either a control protein or NEMO-WT were treated with 10 µM zVAD and then stimulated with 10 ng/ml TNF for 10 hours. The necrosome was isolated by immunoprecipitation with anti-FADD and then immunoblottted for RIP1. A sample of the lysate was blotted for RIP1, NEMO, FADD and GAPDH as a loading control. (E) These data demonstrate that NEMO-null cells preferentially undergo apoptosis and caspase blockade is required for entry into RIP1 kinase-dependent programmed necrosis, which is summarized in the cartoon.
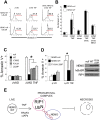
Figure 3. Ubiquitination of RIP1 prevents programmed necrosis.
(A) RIP1-null Jurkat T cells were transduced with either a control protein, RIP1-WT or RIP1-K377R. Cells were pre-treated for one hour with different doses of zVAD and then stimulated with 10 ng/ml TNF for 24 hours. Cell death was quantified by Annexin V staining and flow cytometry. (B) Parental 3T8 Jurkat T cells were transduced with either a control protein or the TRAF2 dominant negative (TRAF2DN). Cells were stimulated with 100 ng/ml TNF for 5 minutes and the TNFR1 complex was immunoprecipitated and subject to western blot with antibodies specific to RIP1. A sample of the lysate before immunoprecipitation (input) was blotted for RIP1 as a loading control. (C) RIP1-null cells were transduced with a control protein (- RIP1) or RIP1-WT (+ RIP1) and then subsequently transduced with a control protein (- TRAF2DN) or the TRAF2DN protein (+ TRAF2DN). All four cell lines were transduced with the IκBαSR. Expression of all transgenes was confirmed by western blot (< marks remaining signal from the TRAF2DN blot) (D) The cells in (C) were pre-treated with 10 µM zVAD for one hour and stimulated with 10 ng/ml TNF. Cell death was measured after 24 hours as described in (A). Representative histograms are shown in (D): the number above the gate indicates the percentage cells that stain with Annexin V after TNF treatment. (E) The bar chart displays the mean percentage of Annexin V staining cells and the SEM of three independent experiments performed as described in (D), * denotes p<0.05. (F) Bar chart shows programmed necrosis in RIP1-null cells reconstituted with either a control protein, RIP1-WT or RIP1-K377R after treatment with 100 nM SMAC mimetic (S) and 10 µM zVAD (Z) for one hour prior to stimulation with 10 ng/ml TNF (T). Cell death was measured after 24 hours as described; the mean percentage of Annexin V staining cells and STD of 3 independent experiments is shown, * denotes p<0.01. All cell lines in Figure 3 express NEMO.
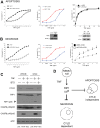
Figure 4. NEMO must be able to bind ubiquitin chains in order to prevent programmed necrosis.
(A) NEMO-null cells expressing IκBαSR were transduced with retrovirus encoding a control protein or NEMO-WT. Cells were pre-treated for one hour with 10 µM zVAD with or without 100 nM SMAC mimetic and then stimulated with 10 ng/ml TNF for 24 h. Cell death was quantified by Annexin V staining and flow cytometry. The number above the gate indicates the percentage of cells that display Annexin V staining in one set of histograms representative of several experiments. (B) The bar chart shows the mean percentage of Annexin V staining cells and SEM from three independent experiments, in this case cells were co-incubated with 30 µM Necrostatin-1 to confirm that the cell death is RIP1 kinase-dependent programmed necrosis. (C) NEMO-null cells were reconstituted with retrovirus encoding NEMO-WT or the NOA/UBAN point mutants NEMO-Y308S, NEMO-F312A or NEMO-L329P, which are unable to bind ubiquitinated RIP1 upon ligation of TNFR1. The mean percentage and SEM of cells that stain with Annexin V after 24 h of 10 ng/ml TNF treatment in the presence of 10 µM zVAD is shown, * denotes p<0.05. (D) NEMO-null cells expressing the IκBαSR were reconstituted with NEMO-WT or NEMO-F312A-L329P, a double point mutation in the ubiquitin-binding domain of NEMO. The mean percentage and SEM of cells staining with Annexin V is shown for three independent experiments with cells co-incubated with the caspase inhibitor q-VD and 10 ng/ml TNF for 24 h, * denotes p<0.05. The immunoblot demonstrates that equivalent amounts of NEMO-WT and NEMO-F312A-L329P protein are expressed. (E) These data demonstrate that cells preferentially undergo apoptosis when NEMO cannot bind ubiquitinated RIP1 and that caspase inhibition is required for these cells to switch to RIP1 kinase-dependent programmed necrosis.
Figure 5. Deubiquitination of RIP1 accelerates both apoptotic and necrotic cell death in NEMO-deficient cells but CYLD is specifically required for programmed necrosis.
(A) Apoptosis of NEMO-deficient 8321 cells was examined by experiments conducted in the presence of 30 µM Necrostatin-1 to prevent necrosis. NEMO null cells were pre-treated with 100 nM SMAC mimetic and 10 µM zVAD for one hour, the line graph shows the mean percentage and SEM from three independent experiments of Annexin V staining cells after 24 h treatment with different doses of TNF, * denotes p<0.05– (left panel). NEMO-null cells were transduced with either a control protein or the TRAF2DN and co-incubated with different doses of TNF for 24 h. The mean percentage and SEM of Annexin V staining cells is shown for three independent experiments, * denotes _p_<0.05. (middle panel). Immunoblot demonstrates expression of TRAF2DN protein. NEMO-null cells transduced with retrovirus encoding non-targeting (shNS) or CYLD-targeting short hairpins (shCYLD) were co-incubated with different doses of TNF for 24h. Cell death was quantified by Annexin V staining and flow cytometry. The mean and SEM of three independent experiments is shown, * denotes _p_<0.05 (right panel). Immunoblot indicates efficient knockdown of CYLD protein in shCYLD cells. Data shown is representative of similar results obtained with two different CYLD-targeting hairpins. (B) Necrosis was examined by conducting a similar series of experiments to those in (A) in the presence of 10 µM zVAD. (C) NEMO-null cells were treated with 100 nM SMAC mimetic in the absence or presence of 100 µM of caspase 8-specific inhibitor IETD and then stimulated with 10 ng/ml TNF. Lysates were blotted with antibody specific for CYLD, RIP1, Caspase 8 and actin as a loading control. The known caspase cleavage product of RIP1 is indicated with an >. The form of cell death that corresponds to the stimuli in each lane is denoted at the bottom of the immunoblot: APOP = apoptosis, NEC = necrosis. (D) Model summarizing cell death data: ubiquitination of RIP1 blocks both apoptosis and necrosis in NEMO-null cells but the deubiquitinase CYLD is specifically required for programmed necrosis to occur.
Figure 6. Recruitment of NEMO to ubiquitinated RIP1 prevents programmed necrosis.
A model that represents the early pro-survival effect of RIP1 ubiquitination is shown. In the first few minutes after ligation of TNFR1, lysine 377 of RIP1 is conjugated to non-degradative ubiquitin chains by the concerted action of the E3 ligases cIAP1, cIAP2 and TRAF2. The bi-partite ubiquitin binding domain of NEMO docks with ubiquitinated RIP1 and this stimulus-specific interaction restrains the kinase domain of RIP1 from instigating cell death by programmed necrosis. In the absence of NEMO, the ubiquitin chains on RIP1 are likely removed by the action of the CYLD deubiquitinase and RIP1 becomes a pro-death signaling molecule that can trigger programmed necrosis when caspase activity is blocked. The pro-survival complex of TNFR1 (I) is the default scenario but a pro-death complex (II) is predicted to arise physiologically when the ubiquitination of RIP1 is prevented by ligation of TNFR2, which triggers degradation of TRAF2, cIAP1 and cIAP2.
Similar articles
- NEMO/IKKgamma regulates an early NF-kappaB-independent cell-death checkpoint during TNF signaling.
Legarda-Addison D, Hase H, O'Donnell MA, Ting AT. Legarda-Addison D, et al. Cell Death Differ. 2009 Sep;16(9):1279-88. doi: 10.1038/cdd.2009.41. Epub 2009 Apr 17. Cell Death Differ. 2009. PMID: 19373245 Free PMC article. - Competitive control of independent programs of tumor necrosis factor receptor-induced cell death by TRADD and RIP1.
Zheng L, Bidere N, Staudt D, Cubre A, Orenstein J, Chan FK, Lenardo M. Zheng L, et al. Mol Cell Biol. 2006 May;26(9):3505-13. doi: 10.1128/MCB.26.9.3505-3513.2006. Mol Cell Biol. 2006. PMID: 16611992 Free PMC article. - Activation of IKK by TNFalpha requires site-specific ubiquitination of RIP1 and polyubiquitin binding by NEMO.
Ea CK, Deng L, Xia ZP, Pineda G, Chen ZJ. Ea CK, et al. Mol Cell. 2006 Apr 21;22(2):245-57. doi: 10.1016/j.molcel.2006.03.026. Epub 2006 Apr 6. Mol Cell. 2006. PMID: 16603398 - TNFR1 signaling kinetics: spatiotemporal control of three phases of IKK activation by posttranslational modification.
Workman LM, Habelhah H. Workman LM, et al. Cell Signal. 2013 Aug;25(8):1654-64. doi: 10.1016/j.cellsig.2013.04.005. Epub 2013 Apr 21. Cell Signal. 2013. PMID: 23612498 Free PMC article. Review. - If the prophet does not come to the mountain: dynamics of signaling complexes in NF-kappaB activation.
Kovalenko A, Wallach D. Kovalenko A, et al. Mol Cell. 2006 May 19;22(4):433-6. doi: 10.1016/j.molcel.2006.05.002. Mol Cell. 2006. PMID: 16713572 Review.
Cited by
- NEMO regulates a cell death switch in TNF signaling by inhibiting recruitment of RIPK3 to the cell death-inducing complex II.
Pescatore A, Esposito E, Draber P, Walczak H, Ursini MV. Pescatore A, et al. Cell Death Dis. 2016 Aug 25;7(8):e2346. doi: 10.1038/cddis.2016.245. Cell Death Dis. 2016. PMID: 27560715 Free PMC article. - Protein-Binding Function of RNA-Dependent Protein Kinase Promotes Proliferation through TRAF2/RIP1/NF-κB/c-Myc Pathway in Pancreatic β cells.
Gao L, Tang W, Ding Z, Wang D, Qi X, Wu H, Guo J. Gao L, et al. Mol Med. 2015 Feb 18;21(1):154-66. doi: 10.2119/molmed.2014.00235. Mol Med. 2015. PMID: 25715336 Free PMC article. - CYLD Proteolysis Protects Macrophages from TNF-Mediated Auto-necroptosis Induced by LPS and Licensed by Type I IFN.
Legarda D, Justus SJ, Ang RL, Rikhi N, Li W, Moran TM, Zhang J, Mizoguchi E, Zelic M, Kelliher MA, Blander JM, Ting AT. Legarda D, et al. Cell Rep. 2016 Jun 14;15(11):2449-61. doi: 10.1016/j.celrep.2016.05.032. Epub 2016 Jun 2. Cell Rep. 2016. PMID: 27264187 Free PMC article. - RIPK1 can mediate apoptosis in addition to necroptosis during embryonic development.
Zhang X, Dowling JP, Zhang J. Zhang X, et al. Cell Death Dis. 2019 Mar 13;10(3):245. doi: 10.1038/s41419-019-1490-8. Cell Death Dis. 2019. PMID: 30867408 Free PMC article. - RIP1 comes back to life as a cell death regulator in TNFR1 signaling.
O'Donnell MA, Ting AT. O'Donnell MA, et al. FEBS J. 2011 Apr;278(6):877-87. doi: 10.1111/j.1742-4658.2011.08016.x. Epub 2011 Feb 8. FEBS J. 2011. PMID: 21232018 Free PMC article.
References
- Hacker H, Karin M. Regulation and function of IKK and IKK-related kinases. Sci STKE. 2006;2006:re13. - PubMed
- Hsu H, Huang J, Shu HB, Baichwal V, Goeddel DV. TNF-dependent recruitment of the protein kinase RIP to the TNF receptor-1 signaling complex. Immunity. 1996;4:387–396. - PubMed
- Devin A, Cook A, Lin Y, Rodriguez Y, Kelliher M, et al. The distinct roles of TRAF2 and RIP in IKK activation by TNF-R1: TRAF2 recruits IKK to TNF-R1 while RIP mediates IKK activation. Immunity. 2000;12:419–429. - PubMed
- Kelliher MA, Grimm S, Ishida Y, Kuo F, Stanger BZ, et al. The death domain kinase RIP mediates the TNF-induced NF-kappaB signal. Immunity. 1998;8:297–303. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous