Assessment of roles for calreticulin in the cross-presentation of soluble and bead-associated antigens - PubMed (original) (raw)
Assessment of roles for calreticulin in the cross-presentation of soluble and bead-associated antigens
Natasha Del Cid et al. PLoS One. 2012.
Abstract
Antigen cross-presentation involves the uptake and processing of exogenously derived antigens and their assembly with major histocompatibility complex (MHC) class I molecules. Antigen presenting cells (APC) load peptides derived from the exogenous antigens onto MHC class I molecules for presentation to CD8 T cells. Calreticulin has been suggested to mediate and enhance antigen cross-presentation of soluble and cell-derived antigens. In this study, we examined roles for calreticulin in cross-presentation of ovalbumin using a number of models. Our findings indicate that calreticulin does not enhance in vitro cross-presentation of an ovalbumin-derived peptide, or of fused or bead-associated ovalbumin. Additionally, in vivo, calreticulin fusion or co-conjugation does not enhance the efficiency of CD8 T cell activation by soluble or bead-associated ovalbumin either in wild type mice or in mice lacking Toll-like receptor 4 (TLR4). Furthermore, we detect no significant differences in cross-presentation efficiencies of glycosylated vs. non-glycosylated forms of ovalbumin. Together, these results point to the redundancies in pathways for uptake of soluble and bead-associated antigens.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
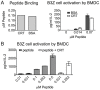
Figure 1. Cross-presentation of a peptide antigen.
(A) 10 µM calreticulin (CRT) or bovine serum albumin (BSA) were incubated with 10 µM peptide (QLESIINFEKLTE-FITC). Free peptide was removed using a centrifugal filter device at 4°C, and peptide still in complex with CRT or BSA was measured (left panel). CRT- or BSA-peptide complexes were incubated with BMDC and B3Z cells. IL-2 production was determined by ELISA of the supernatants after 24 hours (right). Peptide concentration is indicated; CRT or BSA were present at a final concentration of 1 µM. (B) Cross-presentation of free peptide or CRT-peptide complexes was measured as in A. Data are representative of two independent analyses for both A (right panel) and B. Mean ± s.e.m. are shown in A and B.
Figure 2. In vitro cross-presentation of a calreticulin-fused soluble antigen.
(A) Gel-filtration chromatogram of _E. coli_-derived OVA or the OVA-calreticulin (OVA- CRT) fusion protein (left). SDS-PAGE analysis of pooled fractions from left panel; proteins were loaded in equimolar amounts (right) and coomassie stained. (B) Indicated proteins were incubated with BMDC for 3 hours. BMDC were fixed and CFSE labeled OT-I T cells were added. IL-2 levels in supernatants were determined by ELISA (left panel; 24 hour time point). OT-I T cell proliferation was measured at 72 hours in response to 44 µM OVA or OVA-CRT. The solid grey profile indicates the condition where no antigen (no Ag) was added. Data are representative of two independent analyses. (C, D) OVA-CRT and OVA were labeled with allophycocyanin. (C) Labeling intensity was determined by fluorescence imaging of the proteins after separation by SDS-PAGE (inset). Fluorescence intensity was quantified for the indicated proteins. (D) Binding of fluorescent proteins to BMDC was assessed by flow cytometry. BMDC were incubated with labeled proteins on ice before being analyzed by flow cytometry. BMDC not incubated with proteins are depicted as a grey filled. Representative of two independent experiments performed with the same labeled proteins. Mean ± s.e.m. are shown in B.
Figure 3. In vivo cross-presentation of a calreticulin-fused soluble antigen.
(A, B) WT or TLR4−/− recipient mice were injected i.v. with CFSE labeled OT-I T cells. Twenty-four hours later, mice received s.c. injections of the indicated antigen (100 µl of a 220 nM solution). OT-I T cell proliferation was measured 3 days later in the dLN (inguinal). (A) The % of proliferating OT-I T cells averaged from the mice of one experiment is shown in the left panel. Two to three mice were used in all groups. The right panel depicts proliferation in WT recipient mice. (B) Quantification of the % of OT-I T cells of all CD8 T cells recovered in A. Data for A and B are representative of three out of four independent analyses for WT recipients and a single analysis with TLR4−/− recipients. Similar results were obtained in comparisons of OVA and OVA-CRT-induced OT-I proliferation in WT and TLR2/4−/− recipient mice (data not shown). (C) Compilation of the % of proliferating OT-I T cells (left panel) and of the % of OT-I T cells as a function of all CD8 T cells (right panel) from 4 independent experiments performed with WT recipient mice. Two experiments contained 2 doses of antigen and two experiments contained 1 antigen dose. Antigen doses ranged from 0.22 µM–22 µM, using 100 µl. Each point represents the mean of 2–3 mice for that condition. Mean ± s.e.m. are shown in A and B. A two-tailed pair-wise student t-test was used for statistical analyses in C.
Figure 4. Cross-presentation of a particulate antigen.
(A) OVA alone or with calreticulin (OVA+CRT) were conjugated to iron-oxide beads. Levels of OVA conjugated to the beads were quantified using a fluorescence-based assay. (B) The indicated beads were incubated with BMDC for 3 hours. BMDC were fixed and CFSE labeled OT-I T cells were added. Left and middle panels are representative of 1 independent experiment. IL-2 production was determined by ELISA of the supernatants at 24 hours (left panel). OT-I T cell proliferation was measured after 72 hours in response to 10 µl OVA or OVA+CRT beads. The solid grey profile indicates the condition where no antigen (no Ag) was added (middle panel). A compilation of the % of proliferating OT-I T cells from 3 independent experiments is depicted in the right panel in response to 1–5 or 10 µl beads. (C, D) OT-I T cell proliferation and recovery were measured in WT or TLR2/4−/− mice in response to 50 µl beads on day 3. (C) Average proliferation values for 2–3 mice per group (except PBS, where 1 mouse was used) are shown in the left panel. A representative proliferation prolife from WT recipients in response to OVA beads, OVA+CRT beads or PBS (filled in grey) is shown on the right panel. (D) Quantification of the % of OT-I T cells of all CD8 T cells recovered in C. Data are representative of two independent analyses for C. Mean ± s.e.m. are shown in A–D. A two-tailed pair-wise student t-test was used for statistical analysis in B.
Figure 5. In vivo cross-presentation of glycosylated and non-glycosylated OVA.
WT recipient mice were injected i.v. with CFSE labeled OT-I T cells. Twenty-four hours later, mice received s.c. injections of the indicated antigen (2.5–100 µg). OT-I T cell proliferation was measured 3–5 days later in the dLN (inguinal). Left panel: A representative proliferation profile is presented in response to 2.5 µg OVA. Middle and right panels: Two to three mice are averaged to generate each data point, which represents the % of proliferating OT-I T cells (middle) or the % of OT-I T cells as a function of all CD8 T cells recovered (right). Three independent experiments are represented. In one experiment, 2 different OVA (E. coli) preps were used in 2 different groups of mice. In another experiment, two doses (10 µg and 100 µg) of OVA were used in 2 different groups of mice. A two-tailed pair-wise student t-test was used for statistical analysis.
Similar articles
- Major histocompatibility complex class I presentation of peptides derived from soluble exogenous antigen by a subset of cells engaged in phagocytosis.
Reis e Sousa C, Germain RN. Reis e Sousa C, et al. J Exp Med. 1995 Sep 1;182(3):841-51. doi: 10.1084/jem.182.3.841. J Exp Med. 1995. PMID: 7650490 Free PMC article. - Enhanced and prolonged cross-presentation following endosomal escape of exogenous antigens encapsulated in biodegradable nanoparticles.
Shen H, Ackerman AL, Cody V, Giodini A, Hinson ER, Cresswell P, Edelson RL, Saltzman WM, Hanlon DJ. Shen H, et al. Immunology. 2006 Jan;117(1):78-88. doi: 10.1111/j.1365-2567.2005.02268.x. Immunology. 2006. PMID: 16423043 Free PMC article. - Generation of both MHC class I- and class II-restricted antigenic peptides from exogenously added ovalbumin in murine phagosomes.
Muno D, Kominami E, Mizuochi T. Muno D, et al. FEBS Lett. 2000 Jul 28;478(1-2):178-82. doi: 10.1016/s0014-5793(00)01849-4. FEBS Lett. 2000. PMID: 10922492 - Redundancy renders the glycoprotein 96 receptor scavenger receptor A dispensable for cross priming in vivo.
Tewalt EF, Maynard JC, Walters JJ, Schell AM, Berwin BL, Nicchitta CV, Norbury CC. Tewalt EF, et al. Immunology. 2008 Dec;125(4):480-91. doi: 10.1111/j.1365-2567.2008.02861.x. Epub 2008 May 15. Immunology. 2008. PMID: 18489571 Free PMC article. - Calreticulin in the immune system: ins and outs.
Raghavan M, Wijeyesakere SJ, Peters LR, Del Cid N. Raghavan M, et al. Trends Immunol. 2013 Jan;34(1):13-21. doi: 10.1016/j.it.2012.08.002. Epub 2012 Sep 7. Trends Immunol. 2013. PMID: 22959412 Free PMC article. Review.
Cited by
- Antitumor immune responses mediated by dendritic cells: How signals derived from dying cancer cells drive antigen cross-presentation.
Spel L, Boelens JJ, Nierkens S, Boes M. Spel L, et al. Oncoimmunology. 2013 Nov 1;2(11):e26403. doi: 10.4161/onci.26403. Epub 2013 Oct 21. Oncoimmunology. 2013. PMID: 24482744 Free PMC article. Review. - Bioorthogonal protein labelling enables the study of antigen processing of citrullinated and carbamylated auto-antigens.
van Leeuwen T, Araman C, Pieper Pournara L, Kampstra ASB, Bakkum T, Marqvorsen MHS, Nascimento CR, Groenewold GJM, van der Wulp W, Camps MGM, Janssen GMC, van Veelen PA, van Westen GJP, Janssen APA, Florea BI, Overkleeft HS, Ossendorp FA, Toes REM, van Kasteren SI. van Leeuwen T, et al. RSC Chem Biol. 2021 Feb 23;2(3):855-862. doi: 10.1039/d1cb00009h. RSC Chem Biol. 2021. PMID: 34212151 Free PMC article.
References
- Amigorena S, Savina A (2010) Intracellular mechanisms of antigen cross presentation in dendritic cells. Curr Opin Immunol 22: 109–117. - PubMed
- Rutkevich LA, Williams DB (2011) Participation of lectin chaperones and thiol oxidoreductases in protein folding within the endoplasmic reticulum. Curr Opin Cell Biol 23: 157–166. - PubMed
- Nair S, Wearsch PA, Mitchell DA, Wassenberg JJ, Gilboa E, et al. (1999) Calreticulin displays in vivo peptide-binding activity and can elicit CTL responses against bound peptides. J Immunol 162: 6426–6432. - PubMed
- Basu S, Binder RJ, Ramalingam T, Srivastava PK (2001) CD91 is a common receptor for heat shock proteins gp96, hsp90, hsp70, and calreticulin. Immunity 14: 303–313. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials