Randomized trial of radiation therapy plus procarbazine, lomustine, and vincristine chemotherapy for supratentorial adult low-grade glioma: initial results of RTOG 9802 - PubMed (original) (raw)
Randomized Controlled Trial
. 2012 Sep 1;30(25):3065-70.
doi: 10.1200/JCO.2011.35.8598. Epub 2012 Jul 30.
Affiliations
- PMID: 22851558
- PMCID: PMC3732006
- DOI: 10.1200/JCO.2011.35.8598
Randomized Controlled Trial
Randomized trial of radiation therapy plus procarbazine, lomustine, and vincristine chemotherapy for supratentorial adult low-grade glioma: initial results of RTOG 9802
Edward G Shaw et al. J Clin Oncol. 2012.
Abstract
Purpose: A prior Radiation Therapy Oncology Group (RTOG) clinical trial in anaplastic oligodendroglioma suggested a progression-free survival benefit for procarbazine, lomustine, and vincristine (PCV) chemotherapy in addition to radiation therapy (RT), as have smaller trials in low-grade glioma (LGG).
Patients and methods: Eligibility criteria included supratentorial WHO grade 2 LGG, age 18 to 39 years with subtotal resection/biopsy, or age ≥ 40 years with any extent resection. Patients were randomly assigned to RT alone or RT followed by six cycles of PCV. Survival was compared by using the modified Wilcoxon and log-rank tests.
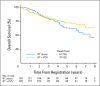
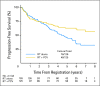
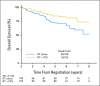
Results: In all, 251 patients were accrued from 1998 to 2002. Median overall survival (OS) time and 5-year OS rates for RT versus RT + PCV were 7.5 years versus not reached and 63% versus 72%, respectively (hazard ratio [HR]; 0.72; 95% CI, 0.47 to 1.10; P = .33; log-rank P = .13). Median progression-free survival (PFS) time and 5-year PFS rates for RT versus RT + PCV were 4.4 years versus not reached and 46% versus 63%, respectively (HR, 0.6; 95% CI, 0.41 to 0.86; P = .06; log-rank P = .005). OS and PFS were similar for all patients between years 0 and 2. After 2 years, OS and PFS curves separated significantly, favoring RT + PCV. For 2-year survivors (n = 211), the probability of OS for an additional 5 years was 74% with RT + PCV versus 59% with RT alone (HR, 0.52; 95% CI, 0.30 to 0.90; log-rank P = .02).
Conclusion: PFS but not OS was improved for adult patients with LGG receiving RT + PCV versus RT alone. On post hoc analysis, for 2-year survivors, the addition of PCV to RT conferred a survival advantage, suggesting a delayed benefit for chemotherapy.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures
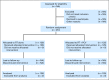
Fig 1.
CONSORT diagram. NA, not assessed; PCV, procarbazine, lomustine, and vincristine; RT, radiation therapy.
Fig 2.
Overall survival for all patients from date of registration/random assignment. PCV, procarbazine, lomustine, and vincristine; RT, radiation therapy.
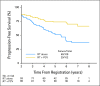
Fig 3.
Progression-free survival for all patients from date of registration/random assignment. PCV, procarbazine, lomustine, and vincristine; RT, radiation therapy.
Fig 4.
Overall survival for patients surviving to 2 years. PCV, procarbazine, lomustine, and vincristine; RT, radiation therapy.
Fig 5.
Progression-free survival for patients surviving to 2 years. PCV, procarbazine, lomustine, and vincristine; RT, radiation therapy.
Comment in
- RTOG 9802: good wines need aging.
van den Bent MJ, Jaeckle K, Baumert B, Wick W. van den Bent MJ, et al. J Clin Oncol. 2013 Feb 10;31(5):653-4. doi: 10.1200/JCO.2012.46.6896. Epub 2013 Jan 7. J Clin Oncol. 2013. PMID: 23295798 No abstract available. - Does RTOG 9802 change practice with respect to newly diagnosed low-grade glioma?
Chamberlain MC. Chamberlain MC. J Clin Oncol. 2013 Feb 10;31(5):652-3. doi: 10.1200/JCO.2012.46.7969. Epub 2013 Jan 7. J Clin Oncol. 2013. PMID: 23295807 No abstract available. - [Conventionally fractioned postoperative radiotherapy up to 50.4 Gy for progressive low-grade gliomas in adults still standard treatment].
Grabenbauer GG. Grabenbauer GG. Strahlenther Onkol. 2013 Apr;189(4):340-1. doi: 10.1007/s00066-012-0293-y. Strahlenther Onkol. 2013. PMID: 23404142 German. No abstract available. - A Randomized Clinical Trial of Radiation With or Without Chemotherapy for Low-grade Gliomas.
Starke RM, Connolly ES, Komotar RJ. Starke RM, et al. Neurosurgery. 2016 Oct;79(4):N17-8. doi: 10.1227/01.neu.0000499709.51090.ea. Neurosurgery. 2016. PMID: 27635971 No abstract available.
References
- Kleihues P, Burger PC, Scheithauer BW. Histological Typing of Tumours of the Central Nervous System. ed 2. Berlin, Germany: Springer; 1993.
- Hinsdale, IL: CBRTUS; 2006. Central Brain Tumor Registry of the United States (CBTRUS): Statistical Report: Primary Brain Tumors in the United States, 1998-2002. http://www.cbtrus.org/reports/2005-2006/2006report.pdf.
- Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2006. CA Cancer J Clin. 2006;56:106–130. - PubMed
- Brown PD, Shaw EG. Low-grade gliomas. In: Gunderson LL, Tepper JE, editors. Clinical Radiation Oncology. ed 2. Philadelphia, PA: Churchill-Livingstone; 2006. pp. 493–514.
- van den Bent MJ, Afra D, de Witte O, et al. Long-term efficacy of early versus delayed radiotherapy for low-grade astrocytoma and oligodendroglioma in adults: The EORTC 22845 randomised trial. Lancet. 2005;366:985–990. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical