Pluripotency in the embryo and in culture - PubMed (original) (raw)
Review
Pluripotency in the embryo and in culture
Jennifer Nichols et al. Cold Spring Harb Perspect Biol. 2012.
Abstract
Specific cells within the early mammalian embryo have the capacity to generate all somatic lineages plus the germline. This property of pluripotency is confined to the epiblast, a transient tissue that persists for only a few days. In vitro, however, pluripotency can be maintained indefinitely through derivation of stem cell lines. Pluripotent stem cells established from the newly formed epiblast are known as embryonic stem cells (ESCs), whereas those generated from later stages are called postimplantation epiblast stem cells (EpiSCs). These different classes of pluripotent stem cell have distinct culture requirements and gene expression programs, likely reflecting the dynamic development of the epiblast in the embryo. In this chapter we review current understanding of how the epiblast forms and relate this to the properties of derivative stem cells. We discuss whether ESCs and EpiSCs are true counterparts of different phases of epiblast development or are culture-generated phenomena. We also consider the proposition that early epiblast cells and ESCs may represent a naïve ground state without any prespecification of lineage choice, whereas later epiblasts and EpiSCs may be primed in favor of particular fates.
Figures
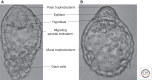
Figure 1.
Bright field images of mature mouse blastocysts showing morphological segregation of epiblast and hypoblast before implantation in the uterus. (A) Peri-implantation at E4.5; (B) diapause. Embryos were freshly flushed from uteri and images captured on an Olympus IX50, with a magnification of 200×.
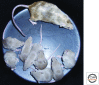
Figure 2.
Adult male mouse ESC chimera generated by injection of 129/Ola ESCs (sandy coat color) into a C57BL/6 blastocyst (black coat color), with pups produced by mating of the chimera to a wild-type albino MF1 female. The gray color of the pups indicates that the sperm arose from colonization of the germline by the injected ESCs.
Figure 3.
Bright field images of embryos just before and after epithelialization of the mouse epiblast at around E5.25. (A) The epiblast is still a compact ball. (B) The epiblast has epithelialized to form a cup-shaped structure and displays a prominent proamniotic cavity. Embryos were freshly dissected free from uteri, deciduum, and Reichert’s membrane and images captured on an Olympus IX50, with a magnification of 200×.
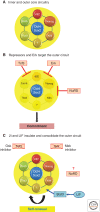
Figure 4.
Illustration of the interconnections between core pluripotency transcription factors and extrinsic stimuli. (A) Schematic of the functionally validated pluripotency factors depicting Oct4 and Sox2 as the essential hub linked to a circuit of factors that are individually more or less dispensable but collectively define and sustain the naïve state. These factors all cross-regulate one another. The question mark signifies a hypothetical additional pivotal self-renewal factor downstream from Stat3. (B) Collapse of the outer circuit of pluripotency factors leads to exit from ground-state pluripotency and ultimately lineage commitment and differentiation. (C). The 2i inhibitors prevent repression of the outer circuit, whereas LIF/Stat3 directly boosts expression of specific factors. How activity of NuRD is modulated in naïve ESCs is currently unclear.
Similar articles
- Generating primed pluripotent epiblast stem cells: A methodology chapter.
Samanta M, Kalantry S. Samanta M, et al. Curr Top Dev Biol. 2020;138:139-174. doi: 10.1016/bs.ctdb.2020.01.005. Epub 2020 Feb 27. Curr Top Dev Biol. 2020. PMID: 32220296 Free PMC article. Review. - Contrasting transcriptome landscapes of rabbit pluripotent stem cells in vitro and in vivo.
Schmaltz-Panneau B, Jouneau L, Osteil P, Tapponnier Y, Afanassieff M, Moroldo M, Jouneau A, Daniel N, Archilla C, Savatier P, Duranthon V. Schmaltz-Panneau B, et al. Anim Reprod Sci. 2014 Sep;149(1-2):67-79. doi: 10.1016/j.anireprosci.2014.05.014. Epub 2014 Jul 1. Anim Reprod Sci. 2014. PMID: 25059199 - Highly Efficient Derivation of Pluripotent Stem Cells from Mouse Preimplantation and Postimplantation Embryos in Serum-Free Conditions.
De Los Angeles A, Okamura D, Wu J. De Los Angeles A, et al. Methods Mol Biol. 2019;2005:29-36. doi: 10.1007/978-1-4939-9524-0_2. Methods Mol Biol. 2019. PMID: 31175643 - Neural stem cells derived from epiblast stem cells display distinctive properties.
Jang HJ, Kim JS, Choi HW, Jeon I, Choi S, Kim MJ, Song J, Do JT. Jang HJ, et al. Stem Cell Res. 2014 Mar;12(2):506-16. doi: 10.1016/j.scr.2013.12.012. Epub 2014 Jan 4. Stem Cell Res. 2014. PMID: 24463498 - Pluripotent cell states and fates in human embryo models.
Sozen B, Tam PPL, Pera MF. Sozen B, et al. Development. 2025 Apr 1;152(7):dev204565. doi: 10.1242/dev.204565. Epub 2025 Apr 2. Development. 2025. PMID: 40171916 Review.
Cited by
- Impairment of DNA Methylation Maintenance Is the Main Cause of Global Demethylation in Naive Embryonic Stem Cells.
von Meyenn F, Iurlaro M, Habibi E, Liu NQ, Salehzadeh-Yazdi A, Santos F, Petrini E, Milagre I, Yu M, Xie Z, Kroeze LI, Nesterova TB, Jansen JH, Xie H, He C, Reik W, Stunnenberg HG. von Meyenn F, et al. Mol Cell. 2016 Jun 16;62(6):848-861. doi: 10.1016/j.molcel.2016.04.025. Epub 2016 May 26. Mol Cell. 2016. PMID: 27237052 Free PMC article. - Efficient Reprogramming of Mouse Embryonic Stem Cells into Trophoblast Stem-like Cells via Lats Kinase Inhibition.
Gao Y, Han W, Dong R, Wei S, Chen L, Gu Z, Liu Y, Guo W, Yan F. Gao Y, et al. Biology (Basel). 2024 Jan 24;13(2):71. doi: 10.3390/biology13020071. Biology (Basel). 2024. PMID: 38392290 Free PMC article. - Dynamic blebbing: A bottleneck to human embryonic stem cell culture that can be overcome by Laminin-Integrin signaling.
Weng NJ, Cheung C, Talbot P. Weng NJ, et al. Stem Cell Res. 2018 Dec;33:233-246. doi: 10.1016/j.scr.2018.10.022. Epub 2018 Nov 2. Stem Cell Res. 2018. PMID: 30458343 Free PMC article. - Capturing Pluripotency and Beyond.
Yeh CY, Huang WH, Chen HC, Meir YJ. Yeh CY, et al. Cells. 2021 Dec 16;10(12):3558. doi: 10.3390/cells10123558. Cells. 2021. PMID: 34944066 Free PMC article. Review. - Harnessing the Therapeutic Potential of Stem Cells in the Management of Chronic Obstructive Pulmonary Disease: A Comprehensive Review.
Singh PV, Singh PV, Anjankar A. Singh PV, et al. Cureus. 2023 Aug 31;15(8):e44498. doi: 10.7759/cureus.44498. eCollection 2023 Aug. Cureus. 2023. PMID: 37711945 Free PMC article. Review.
References
- Basilico C, Ambrosetti D, Fraidenraich D, Dailey L 1997. Regulatory mechanisms governing FGF-4 gene expression during mouse development. J Cell Physiol 173: 227–232 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources